Why do we use steroids, Maddrey's Discriminant Function, and the Lille score in Alcohol-Associated Hepatitis?
We use several calculations to guide our management of Alcohol-Associated Hepatitis (AAH), formally known has Alcoholic Hepatitis. Have you ever wondered why?
Let’s start with a brief review of AAH.
Alcohol-Associated Hepatitis
Alcohol use and death and disability from alcohol use are increasing worldwide, with AAH affecting both men and women, with women more susceptible to alcohol-related liver injury. In the United States, prevalence of alcohol associated liver disease is estimated at around 2% of the general US population with mortality in 2012 estimated at 5.5 per 100,000. Today, the incidence of alcohol associated liver diseases, including AAH, is rising, with recent data during the COVID-19 pandemic demonstrating increased listing for transplant for alcohol-associated liver disease, including a 50% increase in listings for severe AAH. While some transplant centers offer liver transplant for acute severe AAH (a topic that we will discuss in an upcoming post), transplant for AAH is controversial and the condition is associated with a high mortality, with up to 25-40% mortality at one month.
AAH is a distinct manifestation of alcohol-associated liver disease characterized by jaundice and liver failure. The histologic appearance of AAH was first described by Frank Burr Mallory in 1911, however little progress was made until the 1960s. In fact, it was not until the 1960s that alcohol was identified as a causative agent of liver disease. Prior to that, the common belief was that the liver damage seen in heavy alcohol users was instead from malnutrition. Beckett et al introduced the diagnosis of acute alcoholic hepatitis in a 1961 paper, “Acute Alcoholic Hepatitis”, which reviewed 7 case histories. Here, the condition was differentiated from cirrhosis due to alcohol, with the identification of characteristic findings including hepatomegaly, abdominal tenderness, fever, anorexia, nausea, and vomiting. Proposed treatment for AAH was abstinence from alcohol, bed rest, and a high calorie and protein-restricted diet. It was not until the 1970s that corticosteroids were investigated in the treatment of AAH, given the recognition of the role of inflammation in hepatic injury (more on this later).
The Evolution of Nutritional Data in Alcohol Associated Hepatitis
Malnutrition is prevalent in patients with alcohol associated liver disease, with multiple studies demonstrating that virtually every patient with AAH has some degree of malnutrition. Given the widespread early belief that liver disease associated with heavy alcohol use was in fact due to malnutrition, nutrition was amongst the first proposed treatments of alcohol associated liver disease. However, there was quite a debate regarding the composition of the ideal diet for this population.
Patek et al demonstrated in 1948 that a high-protein (140 g) and vitamin B complex supplemented diet in patients with cirrhosis and ascites led to improved survival. In this study, 124 patients were treated with a high protein diet and compared with 368 patients who were treated with the conventional high carbohydrate, low protein, low fat diet and were followed for 10 years, with the survival curve to 5 years shown below.
Despite the initial data supporting a high protein diet, there was a predominant believe that high protein diets precipitated hepatic encephalopathy. One case series of 3 patients with cirrhosis due to alcohol suggested that providing additional protein to these patients (increasing from 50 g to 75 g daily) can induce or aggravate hepatic encephalopathy. The authors also suggested that withdraw of protein may contribute to occasional remission. Low protein diets persisted in the treatment of liver disease well into the 1970s, with the widespread belief that that feeding critically ill patients with liver disease protein, especially to those who may have impaired urea synthesis, created a positive nitrogen balance and aminoacidemia leading to hepatic encephalopathy.
This myth of dietary protein aggravating hepatic encephalopathy was finally debunked in a pivotal 2004 RCT by Córdoba et al. In this study, 30 patients with cirrhosis admitted for hepatic encephalopathy were randomized to receive either a low protein or normal protein diet for 14 days with no difference in hepatic encephalopathy between the two treatment groups.
In a 2016 RCT investigating intensive enteral nutrition in patients with severe AAH treated with corticosteroids, Moreno et al found no significant difference in 6-month mortality between patients treated with intensive enteral feeding and methylprednisolone compared to patients who just received methylprednisolone. However, low daily calorie intake was associated with higher morality and, conversely, improved 6-month survival was associated with ≥65 g/d of lipids and ≥77.6 g/d of protein intake. On closer look, there was a non-significant lower mortality rate at 1 and 6 months in the intensive nutrition group compared to the control. Interestingly, enteral feeding was difficult to implement, with nearly half of patient in this group withdrawing from the feeding tube prematurely, which may have contributed to the negative trial results.
To summarize, nutrition has always played a pivotal role in the treatment of AAH, with more recent data demonstrating that high calorie high protein diets are associated with improved outcomes. In fact, some of the high mortality rates seen in the early trials discussed below may partially be due to poor nutrition (for example, Maddrey restricted protein to 20 g/d in patients with hepatic encephalopathy).
Why are steroids used in Alcohol Associated Hepatitis?
Corticosteroids are used to treat a myriad of chronic inflammatory diseases. Steroids were first used in the 1950s to treat cirrhosis, with the aim of decreasing fibrosis and inflammation. In 1950, Webster et al treated 9 patients with cirrhosis with adrenal cortex extract in hopes of “altering metabolism of carbohydrate and protein so as to protect the liver and encourage reparative process.” Remarkably, all patients survived with improved liver associated markers and “no evidence of liver dysfunction” at 1 year follow up.
In the years that followed, steroids were used to treat cirrhosis in France with conflicting results reported in case-reports and studies. In 1960, Wells et al performed a randomized control trial of prednisolone and testosterone propionate in 97 patients with cirrhosis. Patients treated with prednisolone and corticotrophin had a 26% mortality compared with 55% mortality in controls and 31% mortality in patients treated with large doses of testosterone as seen in the following table.
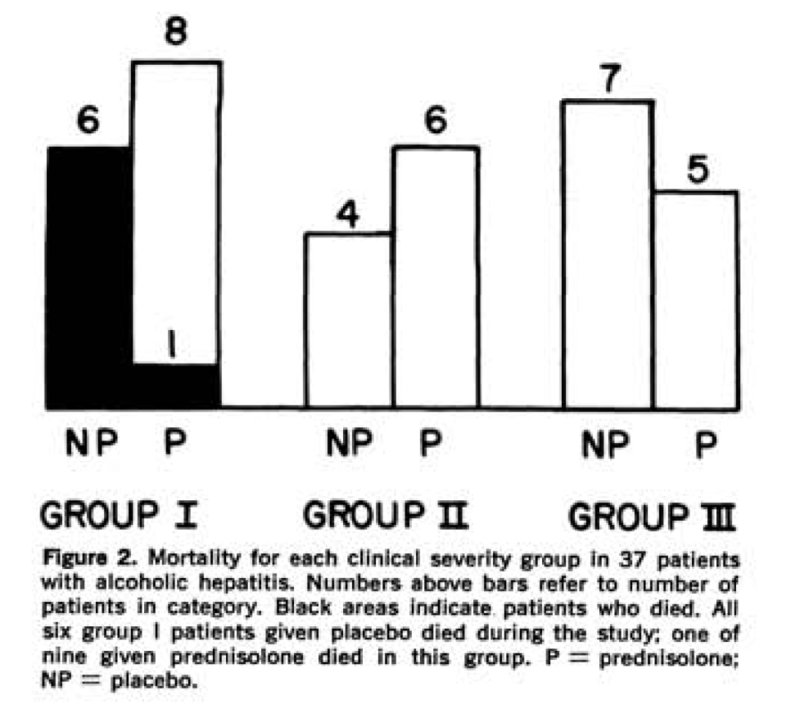
Given reports of improved mortality with prednisolone in cirrhosis, in 1971, Helman et al randomized 37 patients with biopsy-proven AAH to receive prednisolone 40 mg daily or placebo. Patients were classified into groups based on clinical disease severity based on presence of hepatic encephalopathy, with group I patients severely ill with evidence of hepatic encephalopathy during the first 10 days of admission, group II moderately ill but no evidence of encephalopathy, and group III mildly ill. Mortality was only seen in group I patients with only one death seen in those treated with prednisolone (p<0.01), represented in the following figure. Interestingly, patients in group I and II treated with prednisolone also had a statistically significant increase in daily caloric intake.
Porter et al also studied prednisolone 40 mg daily in a randomized control trial of 20 patients with severe AAH, with 11 patients receiving prednisolone. In this study, 45% of patients in the treatment arm survived to discharge compared to only 22% of controls, however this difference was non-statistically significant (see figure below).
These early studies showing the possibility of survival benefit of corticosteroid use in AAH laid the foundation for Maddrey’s famous study.
How was Maddrey’s Discriminant Function developed?
The Maddrey’s Discriminant Function (DF) is used to evaluate AAH severity and mortality and is used to guide treatment with corticosteroids. In the seminal double blind randomized trial in 1978 from Maddrey el al, 55 patients were randomized to receive 28 to 32 days of prednisolone or placebo, with findings of a significant reduction in early mortality when patients were treated with corticosteroids. Of the 24 patients who received prednisolone, only 1 patient died during the study period (p=0.1), with 2 additional patients in this group dying within 48 days of completing the study. Of the 31 patients who received placebo, 4 patients died during the study with an additional 2 patients dying within 5 days of study completion. All deaths occurred in patients with severe disease, with 6/18 (33%) dying in the placebo group versus 1/13 (6%) of the group treated with prednisolone (p=0.1). Factors associated with fatal outcomes were identified, and a discriminant function analysis was performed using laboratory markers. The DF formula that best predicted survival was DF= 4.6 x prothrombin time (sec) + serum bilirubin (mg/dL). Using this formula, all deaths occurred in patients whose initial DF was >93, as seen in the following figure. Also, using the cut off DF >93, treatment with corticosteroids was found to significantly improve mortality (p=0.03).

So then how did we come up with a Maddrey’s DF cutoff of 32?
In a 1989 multi-centered trial on methylprednisolone therapy in patients with severe AAH, the DF was later modified to accommodate for PT values at different centers, with the formula as we now know it modified to DF= 4.6 (prothrombin time-control time) + serum bilirubin, where severe disease was defined as a DF≥32 or the presence of hepatic encephalopathy. In this trial, where patients with severe disease were randomized to receive methylprednisolone or placebo, only 2/35 patients (6%) given methylprednisolone died within 28 days of randomization, whereas 11/31 patients (35%) given placebo died. Today, Maddrey’s DF is used to identify patients at high risk of short-term mortality (DF ≥32) who may benefit from treatment with corticosteroids.
Why do we use prednisolone instead of prednisone?
As many of us have been taught, prednisone is a prodrug that is converted to prednisolone by the 11β-HSD1 enzyme, which occurs primarily in the liver but also in muscle and fat cells. Powell et al measured serum prednisolone levels after administering prednisone or prednisolone in 22 patients with acute or chronic liver disease. He found that in patients with “active liver disease”, defined as elevated bilirubin or transaminase, prednisolone levels were significantly higher in patients who received prednisolone as compared to prednisone, suggesting incomplete conversion of prednisone to prednisolone in patients with liver injury. However, this effect was only appreciable the first few hours after administration and drug levels were only measured to 6 hours as seen in the figures below.

Powell’s findings were not reproducible with subsequent studies finding no major defect of prednisone metabolism in patients with active liver disease. Furthermore, the bioavailability of oral prednisone in patients with liver disease is nearly 100%, with effective conversion to prednisolone in this patient population.
So, while the answer as to why we use prednisolone over prednisone is answered above, the truth is that using prednisolone over prednisone offers little advantage, especially when it is frequently more difficult and costly to obtain prednisolone over prednisone.
Why are steroids the last treatment standing?
As discussed above, steroids have been used to treat AAH since in the 1970s and, along with supportive care, remain the mainstay of treatment. Pentoxifylline, a non-selective phosphodiesterase inhibitor known to decrease TNF gene transcription, was previously recommended to treat AAH. Akriviadis et al performed a prospective, randomized, double blind trial of pentoxifylline in patients with AAH. In this study, 49 patients received pentoxifylline and 52 received placebo, with results showing improved survival in patients receiving pentoxifylline versus placebo (24.5% v 46% mortality, p=0.037). Pentoxifylline was also shown to decrease hepatorenal syndrome (HRS) as a cause of death—only 50% of the deaths in patients treated with pentoxifylline were from HRS, whereas 92% of deaths in the placebo group were from HRS. While this initial study showed a markedly improved survival with pentoxifylline, the study size was relatively small and pentoxifylline was not compared with corticosteroids.
The comparison between pentoxifylline and corticosteroids was not made until 2015 in the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial. In this multicenter, randomized, double blind trial, 1103 patients with a clinical diagnosis of AAH were randomized into one of four treatment groups: placebo-placebo, prednisolone-placebo, pentoxifylline-placebo or prednisolone-pentoxifylline. The primary outcome was mortality at 28 days, with secondary outcomes of mortality or liver transplantation at 90 days and at 1 year. At 28 days, 17% of the placebo-placebo group had died, 14% in the prednisolone-placebo, 19% in the pentoxifylline-placebo, and 13% in the prednisolone-pentoxifylline group. Neither prednisolone nor pentoxifylline decreased mortality at 90 days or 1 year, with Kaplan-Meier survival curves below.
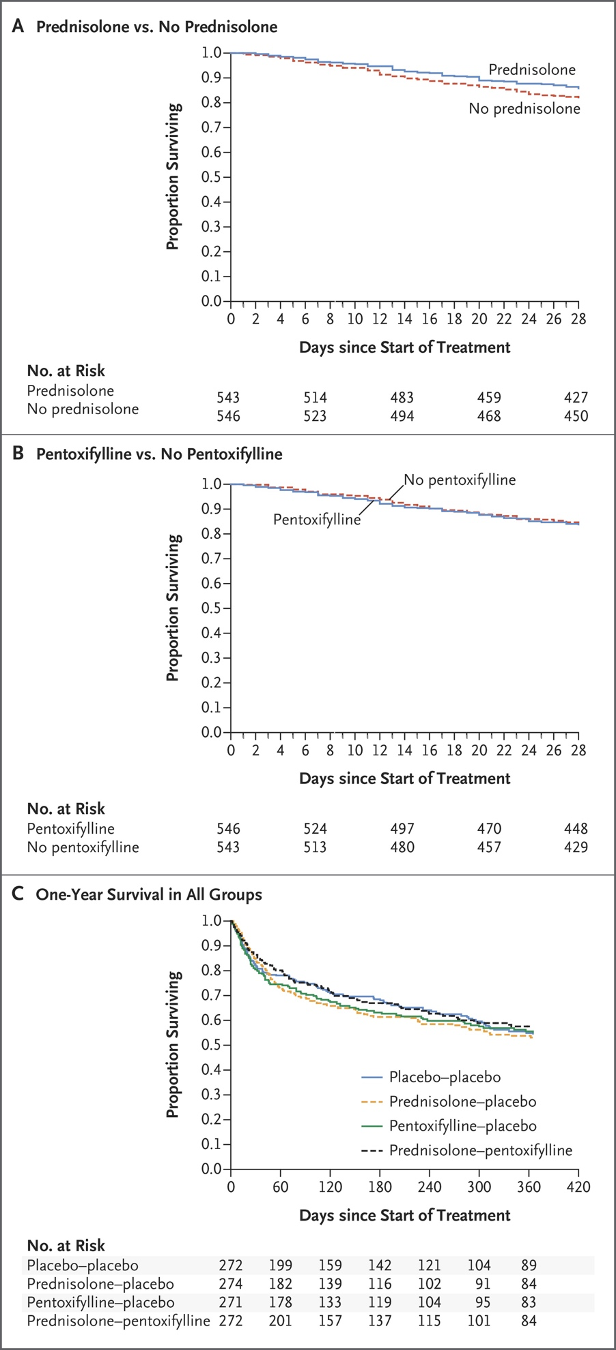
In summary, the results demonstrated the mortality benefit of prednisolone at 28 days, but not at 90 days or 1 year, with no significant difference in outcomes with the addition of pentoxifylline. This seminal study led to changes in practice and, in the Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Guidance from AASLD, pentoxifylline is no longer recommended for treatment of AAH.
N-acetylcysteine NAC) has been studied in AAH, with combination treatment of NAC and prednisolone showing improved survival at 1 month but not at 6 months when compared to patients just treated with prednisolone (8% v 24%, p=0.006). While NAC makes it into the guidelines as a treatment of potential benefit, further validation is needed.
Other treatments that have been studied in AAH but were not found to be beneficial were infliximab (study was actually halted by sponsor given non-significant trend towards increased mortality), etanercept, insulin and glucagon, propylthiouracil, and antioxidants such as vitamin E and S-adenosylmethionine.
Why don’t we give steroids to everyone?
Corticosteroids have long been associated with increased risk of infection. A 2009 prospective study including 246 patients with severe AAH by Louvet et al explored incidence of infection in patients with severe AAH (DF ≥32) before and after treatment with steroids (all patients received prednisolone). In this study, 25.6% of patients had infections identified at admission and 23.7% developed infection after treatment with steroids. Patients infected after treatment with corticosteroids had significantly lower 2-month survival, with Kaplan-Meir survival curves below.
In this study, the Lille Model, which we will discuss next, was used to identify corticosteroid non-responders, with infections occurring more frequently in this group than corticosteroid responders, 42.5% v 11.1% (p<0.000001), respectively. So, while steroids can be beneficial in a subset of patients, there is a significant risk of infection, especially in those who do not initially respond to treatment.
Another study retrospectively analyzed data from 1092 patients from the STOPAH trial to compare effects of infection on clinical outcomes. Infections classified as more serious occurred more frequently in patients treated with prednisolone (p=0.002), with more patients treated with prednisolone developing infections after treatment than those not treated with prednisolone (p=0.024). Development of an infection in patients treated with corticosteroids was associated with increased 90-day mortality, independent of MELD or Lille scores (p=0.002). This study also found that high levels of circulating 16s ribosomal bacterial DNA predicted infection within 7 days of prednisolone therapy (p=0.001), a finding that was not seen in patients who did not receive prednisolone. Given these findings, the authors proposed that levels of circulating bacterial DNA could identify patients at high risk of developing infection with corticosteroids.
Apart from patients with active infections, steroids should not be given to patients with more mild presentations who are expected to recover on their own (DF<32, MELD <21), patients with gastrointestinal bleeding, pancreatitis, acute kidney injury, and active uncontrolled infections.
Lille Model
Given steroids are associated with increased risk infection, the Lille Model was developed to assess response to steroids in order to guide treatment duration. The Lille Model score can be calculated here. It is a combination of six reproducible variables with a high sensitivity and specificity for early identification of patients treated with steroids for AAH at high risk of death at 6 months. The initial study in 2007 included patients with biopsy-confirmed severe AAH defined as a DF ≥32 or hepatic encephalopathy. Patients were treated with prednisolone 40 mg daily for 28 days (or methylprednisolone 32 mg daily if unable to take oral). The model was developed using data from 320 patients with severe AAH. Patients were followed for at least 6 months, with the primary endpoint being survival at 6 months. Variables associated with survival and mortality were analyzed with univariate analysis. The variables found to be statistically significant were then added to a logistic regression model. The final logistic regression function (R Lille model) combined the following six variables: age in years, albumin day 0 in g/L, evolution in bilirubin level in μM, renal insufficiency, bilirubin day 0 in μM, prothrombin time in seconds.
The model was then validated prospectively in 118 patients and compared against the MELD, Maddrey, and Glasgow Alcoholic Hepatitis Score (GAHS) scores where it was found to have a significantly higher AUROC, meaning the Lille Model outperformed other prognostic models. Patients with a Lille score of ≥ 0.45 had a markedly decreased 6-month survival (25% ± 3.8% versus 85% ± 2.5%, P < 0.0001), with the 0.45 cut off identifying 75.6% of the observed deaths. See the Kaplan-Meier survival analysis below using the 0.45 cutoff of the Lille model.
Using the 0.45 Lille model cutoff, nearly 40% of patients were identified as steroid non-responders. The absence of mortality benefit at 7 days in patients with Lille score ≥0.45 with no difference in outcomes between patients given steroid versus placebo suggests that continuing corticosteroids at 7 days in this subset of patients may not provide benefit. Given that subsequent studies have demonstrated that steroid-non responders are at greater risk of infection, continuing steroids in this population does not improve outcome and may be harmful.
Can we use the Lille Model with 4-day bilirubin?
But what about patients at high risk of infection where 7 days may be too long to wait? Can we calculate the Lille score after only 4?In 2017, Garcia-Saenz-de-Sicilia et al performed a multinational retrospective analysis where the Lille Model was calculated at day 4 and at day 7 in patients treated with prednisolone. The primary end point of the study was all cause mortality at 90 days from hospital admission. Of 260 patients with a DF≥32 on admission, 163 were treated with corticosteroids. The Lille model was calculated using day 4 and day 7 bilirubin. The day 4 score (LM4) was calculated in 153 (94%) patients and the day 7 score (LM7) in 150 (92%) of patients. There were 70 (45.8%) non-responders using LM4 and 68 (45.3%) with LM7, with correlation between 28 and 90-day mortality between the scores and a 91.1% agreement between LM4 and LM7 to predict response to therapy (p<0.001). Of the 7 patients who were non-responders by day 4 and responders by day 7, 71% died. This study suggests that the Lille Model can be calculated at day 4 of corticosteroid treatment to accurately identify patients who are corticosteroid non-responders in order to minimize steroid use in this population who is particularly susceptible to infection.
What about other scoring systems?
While Maddrey’s DF is still widely used to model prognosis as well as to guide steroid use, it has its drawbacks. Namely, the DF uses PT which can vary significantly between laboratories, and the initial validation of mortality data was performed on patient cohorts from more than 50 years ago. Newer prognostic models have been developed, including the MELD score, AIBC score, and GAHS. So, which scores have the best performance?
The GAHS was formulated using variables associated with increased mortality (age, serum bilirubin, blood urea, prothrombin time, and white blood cell count). A cut off of ≥9 was suggested to define poor prognosis. The GAHS had an 81% accuracy when predicting 28-day outcomes compared to DF ≥32, which had an overall accuracy of 49%.
The, age, serum bilirubin, international normalized ratio and serum creatinine (AIBC) score was developed and validated in a Spanish cohort of patients with biopsy-proved AAH treated with corticosteroids. It stratifies patients into low, intermediate, and moderate risk of mortality at 90 days and one year.
The MELD score, which you can read more about in this Why? series article, can also be used to assess prognosis in AAH. In a retrospective cohort of 73 patients with AAH, a MELD ≥21 was comparable to DF ≥32 in predicting 30-day and 90-day mortality, with no statistically significant differences. See the receiver operating characteristic curves below comparing MELD with DF.
Louvet et al studied combining static and dynamic models to develop a better method of predicting outcomes in AAH. They used data from several databases of patients with severe AAH and compared performance of Maddrey + Lille, MELD + Lille, AIBC score + Lille, finding that the MELD + Lille outperformed other combinations. The following figure shows the 2- and 6-month overall mortality probability using the joint MELD + Lille Model, with the “+” indicating a hypothetical patient with a MELD of 21 and Lille of 0.45 showing a 15.3% mortality at 2 months and a 23.7% mortality at 6 months.
Forrest et al investigated prognostic models in AAH in patients from the STOPAH trial, comparing the DF, GAHS, AIBC score, MELD score, and Lille Model. They found that the GAHS, AIBC, and MELD (MELD<25) scores were superior to the DF in determining mortality risk, with the Lille providing the best prognostic capability of dynamic scores.
Arab et al recently aimed to retrospectively identify the range of MELD scores where steroids were most effective for AAH. They included data from 3,380 patients, of which 1225 patients received corticosteroids. They found that steroids only increased survival in patients with MELD scores between 21 and 51, with the most survival benefit seen in patients with MELD scores between 21 and 39 (p<0.001) as seen in the following figure:
Hot off the press, Morales-Arráez et al examined the accuracy of different scores to predict short term mortality in AAH in 2,581 patients at 85 tertiary care centers in 11 countries. Using this global patient population, they found that the MELD score had the best performance in predicting short term mortality. They found that the Glasgow and AIBC scores also outperformed DF, as seen in the ROC curves below.
In conclusion, steroids have remained the mainstay of pharmacologic treatment for severe AAH since the 1970s. There are multiple prognostic models that can be used to identify patients with high mortality who may benefit from steroid treatment, with recent literature suggesting that the MELD or the combination of MELD + Lille may be the best prognostic model. However, as steroids only provide short-term survival benefit and a significant subset of patients with severe AAH do not respond to steroids, development of treatment providing long-term benefit is paramount.
Key Points
- Use Maddrey's DF or MELD to determine severity of AAH and possible need for corticosteroids, with DF≥32 and MELD≥21 indicating high risk of short-term mortality and possible benefit of corticosteroids
- Corticosteroids provide a short-term mortality benefit, but do not improve mortality at 90 days or 1 year
- Steroids are associated with increase in infection, with steroid-non-responders at even higher risk of infection
- The Lille model with either bilirubin at 4 or 7 days can be used to identify steroid non-responders so as to minimize steroid use in this population
- Use combination MELD + Lille for most accurate prognostication