Why would we consider plasma exchange in Acute Liver Failure and Acute-on-Chronic Liver Failure?
Why do we need TPE in liver disease?
Acute liver failure (ALF) and acute-on-chronic liver failure (ACLF) are associated with high mortality unless treated appropriately.1 Therapeutic plasma exchange (TPE) may be used in ALF and ACLF either as a “bridge” to liver transplantation (LT) or as a part of medical management in patients who are unfit for LT.2
TPE is a process by which the plasma of an individual is filtered through an extracorporeal circuit which helps remove endotoxins, cytokines, and damage-associated molecular patterns (DAMPS), which are then replaced by fresh frozen plasma (FFP) alone or in combination with plasma expanders.3,4 By removing these cytokines, toxins, and autoantibodies, TPE provides the native liver a chance to “rest and recover”.
In this review, we explore the mechanisms that contribute to the efficacy of TPE in patients with liver disease and its outcomes reported in literature.
History of exchange transfusions and TPE in liver disease
Initial attempts at utilization of exchange transfusion in liver failure dates back to 1949 (Bessis et al.), although they were unsuccessful. The first successful use of TPE was in 1952 by Beekman et al in a 13 year-old child. Subsequently, exchange transfusion was attempted in multiple smaller cohorts (Trey et al. [1966], Jones and Sherlock [1967], Hecht [1967]) in which improvement in survival was reported. From 1958-1970, there were reports of almost 97 patients receiving exchange transfusions, of whom 33 (34%) survived. However, in most of these studies, complete recovery was seen in a limited number of patients and most succumbed to delayed complications such as infections. This encouraged the first clinical trial of exchange transfusion in 1973 by Redeker et al., in 28 patients with fulminant liver failure which failed to show any survival benefit of exchange transfusion. This stemmed the initial enthusiasm around exchange transfusions and alternative techniques such as hemoperfusion and other bio-artificial devices began to be explored.
How does TPE work in acute liver failure?
Impact on disease pathogenesis
Majority of ALF are precipitated by viral agents, drugs or toxins.1 Pathogen associated molecular patterns (PAMPs) and DAMPs produced due to hepatic injury stimulates the immune system to produces pro-inflammatory cytokines, tumor necrosis factor (TNF)-𝛂, interleukins (IL-6, IL-10, IL-1β) and reactive oxygen species (ROS). These cytokines may lead to an uncontrolled immune response resulting in secondary organ injury, endothelial dysfunction, cerebral edema, microvascular injury and monocyte dysfunction.5 In such a clinical setting, TPE removes the offending cytokines and DAMPs from the plasma which restores vascular tone, prevents endothelial dysfunction, maintains adequate organ perfusion and helps ameliorate the immune response.
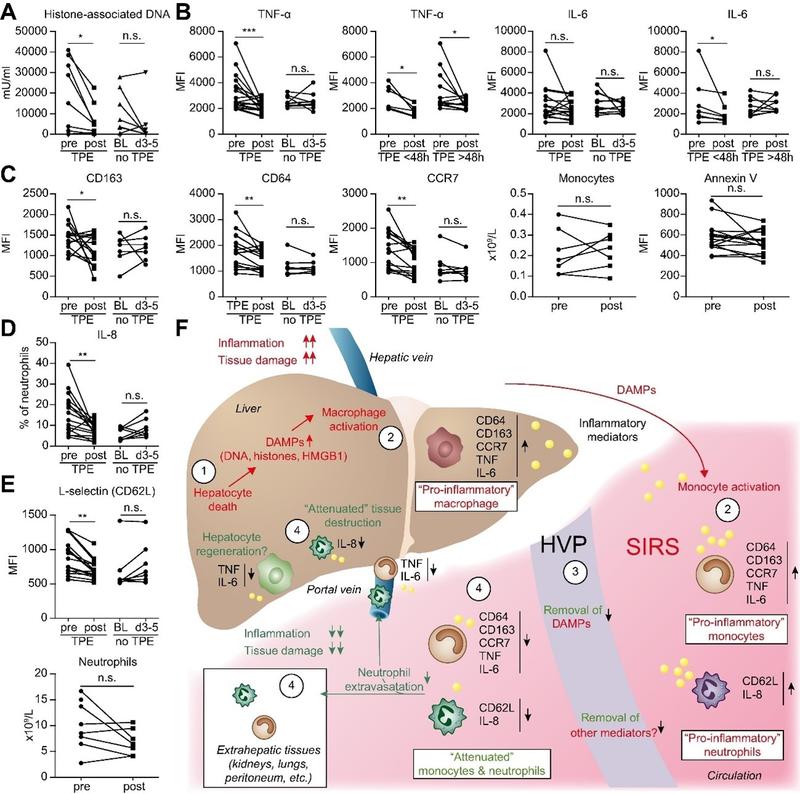
In a large multicentric randomized trial from Europe, Larsen et al. demonstrated the improvement in vascular tone and endothelial dysfunction in ALF with TPE (Figure 1). Patients receiving TPE had higher rates of clearance of DAMPs, angiopoietin-2 and restored functionality of monocytes and neutrophils. This would clinically translate to maintenance of circulatory function, maintenance of systemic and cerebral perfusion pressures along with lower rates of encephalopathy.6 They also demonstrated a reduction in pro-inflammatory cytokines, with an increase in anti-inflammatory cytokines (IL-10, IL-4 and TGF-β) in patients receiving TPE, without affecting the number or functionality of lymphocytes. Nakamura et al. and Maiwall et al., also reported significantly lower levels of circulating pro-inflammatory cytokines in patients who received TPE as compared to those who did not, leading to abatement of the immune response and prevention of development of secondary organ failures.7,8 Induction of anti-inflammatory response would potentially prevent immune exhaustion associated with ALF and reduce the rates of acquired infections seen in the later stages of management of ALF patients.
Figure 1. Pathogenesis of ALF and changes in inflammatory markers with therapeutic plasma exchange as compared to standard medical therapy (adapted from Larsen et al).6 This figure provides an overview of the major cytokines and mediators of hepatic injury in ALF as well as the impact of TPE on these mediators.
Impact on clinical outcome
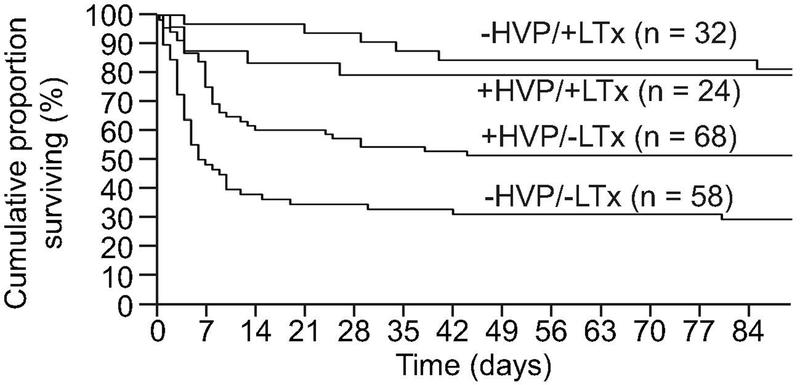
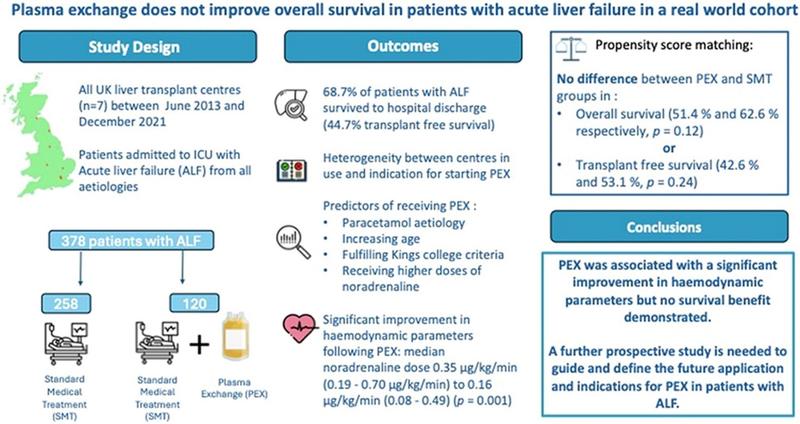
Larsen et al., reported improved overall survival with among patients who were unsuitable for LT (58.7%) as compared to those receiving SMT alone (47.8%) (Figure 2).6 However, for patients receiving an LT, performing TPE as a “bridge to transplant” did not lead to higher survival rates as compared SMT (CI 0.37 to 3.98; p=0.75). Patients receiving TPE also had lower vasopressor requirement, serum bilirubin and INR as compared to those receiving SMT, although the intracranial pressure in both groups were observed to be similar during the first week of ICU stay. Patients receiving TPE also had lower CLIF and SOFA scores during their ICU stay as compared to those receiving SMT. This suggests that TPE helps in recovery of liver functions, reduces systemic inflammation and improves circulatory functions in patients with ALF as compared to SMT. In a similar study from India, Maiwall et al., also reported improvement in 21-day survival using standard volume TPE (SVP) as compared to SMT.8 The choice of HVP or SVP in these studies were empirical and there is a lack of evidence regarding which should be preferred in practice. Improvements in survival have also been reported in smaller observational studies by Hung et al and Nakamura et al.7,9 A recent meta-analysis reports that TPE improves 30-day survival in ALF by 41% and overall survival by 35%.10 In contrast, a recent multicentre cohort study from UK corroborated the improvements in clinical parameters reported by Larsen et al., such as MELD, serum lactate levels, hemodynamic instability and pH with TPE but failed to show an improvement in survival as compared to SMT (TPE: 51.4% vs 62.6% with SMT, p=0.12) (Figure 3).2 This study highlighted the lack of standardization in the protocols of TPE, time of onset and the need for the development of prognostic scoring systems which would help in early identification of those who need and benefit from TPE.
Figure 2. Outcomes of ALF patients in the study by Larsen et al., stratified by use of HVP and LT.6 Patients who received LT did not have a survival benefit from receiving additional sessions of TPE, however, for patients who did not receive LT, there was a definite survival benefit.
Figure 3. Results from the multicentric study by Burke et al.2 In contrast to Larsen et al.,6 this study reports that TPE does not improve overall survival or transplant free survival in ALF despite improving hemodynamic parameters, pH and lactate.
Would TPE work in acute-on-chronic liver failure?
DAMPs, immune dysfunction, coexisting gut dysbiosis, endotoxemia and cholemic organ injury contribute to the systemic inflammation in patients with ACLF.11,12 Moreau et al., recently reported a 38-metabolite blood fingerprint specific for patients with ACLF, and reported that higher grades of organ failures are associated with greater systemic inflammation. Thus, strategies aiming to reduce systemic inflammation can help abate the progression of ACLF.
Impact on disease pathogenesis
TPE dampens systemic inflammation and improve hepatocyte regeneration by removing DAMPs, cytokines, bile acids and endotoxins. Studies from China and India have demonstrated a reduction in serum concentrations of IFN-γ, interleukins and TNF-𝛂 among patients of ACLF undergoing TPE as compared to SMT.13,14 Mao et al. reported that a decline in dysfunctional Tregs due to TPE was associated with better survival rates than those who had high Tregs.15 The reduction in pro-inflammatory cytokines helps control the systemic inflammation which is the primary driver of outcomes in ACLF. It prevents the development of further organ failures and immune fatigue. Patients who respond to TPE have been shown to have an improved monocyte phagocytic function, mitochondrial respiration and increased anti-inflammatory IL-1 receptor antagonist (IL-1RA) as compared to non-responders.13 The improved leucocyte function may help in effective clearance of infective agents and prevents development of multi-organ dysfunction. Modulation of gut dysbiosis with TPE is an interesting concept as disruption of the gut-liver axis and translocation of enteric pathogens may precipitate ACLF and subsequent organ failures. TPE may help in removing endotoxins and reducing the inflammatory response to the enteric pathogens and aid in recovery. Renal failure is one of the most common organ failures in ACLF and cholemic nephropathy is believed to play a contributory role. TPE may prevent the development of cholemic nephropathy by clearing bile salts from the circulation, thus protecting the kidneys from injury. However, it is pertinent to note here, that despite being theoretically attractive concepts, the role of TPE in preventing gut dysbiosis or cholemic nephropathy is yet to be demonstrated in prospective studies.
Impact on clinical outcomes
Ye et al., demonstrated a greater in-hospital reduction in levels of serum ammonia among survivors of ACLF who received TPE (from 116.8 ± 36.3 µ/dL to 44.8 ± 16.3 µ/dL) as compared to non-survivors on SMT (105.7 ± 30.2 µ/dL to 57.1 ± 20.3 µ/dL).16 In a prospective study of 280 patients with Hepatitis B virus related ACLF, Yu et al., demonstrated that patients with a MELD score between 30-40 who received TPE had better 90-day survival (50.6%) than those who received SMT (13.9%).17 However, outcomes were similar in patients with MELD>40. In a smaller observational study, Mao also reported better 90-day survival among those receiving TPE (41.9%) as compared to SMT (25.2%).15 Similar to Yu et al., they also reported improved survival in patients with lower MELD scores (20-30) as compared to those who had higher scores (>30). Subsequently, Chen et al., in a multicentric study from China reported that delaying initiation of TPE (due to infections, bleeding, HRS and advanced HE) may lead to suboptimal outcomes in patients with ACLF.18 Based on these studies, the APASL proposes a narrow therapeutic window of 2 weeks in the early stages of ACLF (“golden-window”) to initiate TPE in ACLF, for preventing MOF and improving survival.19 A recent meta-analysis reports that TPE is more effective in the short term, improving 30-day survival in ACLF by 36% and 90-day survival by 21%.10 While the initial results from these studies are encouraging, the results should be cautiously interpreted as they predominantly relate to a single etiology with a limited study population. A recent meta-analysis shows that infections are the most common precipitants of ACLF and TPE may be contraindicated in such situations as it may worsen sepsis by removing immunoglobulins from blood. Thus, more robust data and clinical trials are required before TPE may be definitively recommended for ACLF and its use is presently restricted to a case-based approach.
Combination therapy with TPE in liver disease
One of the major drawbacks of TPE include its inability to remove substances <50,000 kDa and its non-selective nature which leads to removal of albumin and immunoglobulins. Double plasma membrane absorption system (DPMAS) uses a plasma filter to continuously remove plasma and can remove medium-sized and macromolecular toxins without causing significant changes in albumin.20 Yao et al. reported that combining DPMAS with TPE significantly improves survival (57.4%) compared to TPE alone (41.7%, p=0.04).21 Similar studies combining TPE with hemoperfusion or continuous hemodiafiltration have shown improvements in 90-day survival as compared to SMT (60 vs 47%).22 A recent meta-analysis of DPMAS with TPE revealed greater improvement in bilirubin and INR when TPE is combined with DPMAS along with lower requirement of FFP.23 A major limitation of these studies is that they have been predominantly performed in patients with Hepatitis B related ALF/ACLF and require validation in larger cohorts with more diverse etiologies.
Adverse effects
Prior studies on TPE have reported both major (0-3%) and minor adverse effects (8-31%) of TPE.24 The most common adverse events include hypocalcemia (tingling, perioral numbness, tetany), fever, urticaria, hypotension (most commonly due to replacement fluid), nausea, catheter-related sepsis and fever. The use of FFP carries a risk of allergic reactions, while heparin may lead to thrombocytopenia in 0.5-1.5% patients undergoing TPE. Hypocalcemia is often attributed to the use of citrate and may be reduced by targeting a citrate concentration of 1.0-1.8 mg/kg/min.25 In the study by Larsen et al., cardiac arrythmias, pancreatitis, ARDS, infections and hemorrhage were common adverse events but their incidence in the TPE arm was similar to that in the SMT arm. In contrast, Maiwall et al, reported the most common encountered adverse events to be thrombocytopenia and sepsis.8,13
Conclusion
- TPE reduces the inflammatory burden in patients with ALF and ACLF and may prevent MOF when initiated early in the disease course
- Majority of studies support the role of TPE in improving survival among patients with ALF
- There is paucity of granular data on effects of TPE on outcomes of ACLF
- Combination of TPE with DPMAS or other filtration techniques may improve its efficacy while reducing its adverse effects.