Why shouldn’t ammonia be used in the diagnosis and management of hepatic encephalopathy?
Hepatic encephalopathy (HE) is one of the major reasons patients with cirrhosis are admitted to the hospital. Often before they have been seen by the gastroenterology or hepatology team, a serum ammonia level is measured. In fact, in one single-center study, 95% of patients admitted for HE had an ammonia level checked on admission. AASLD guidelines recommend that hepatic encephalopathy is a clinical diagnosis, stating “Increased blood ammonia alone does not add any diagnostic, staging, or prognostic value for HE in patients with CLD”. So, what do we do with this ammonia level?
In this post, we will explore the history of hepatic encephalopathy and its relationship to ammonia, the situations in which an ammonia level may or may not be useful, and the limitations in ammonia measurement.
Why do we care about HE?
Hepatic encephalopathy marks a transition point in the natural history of cirrhosis. As many as 40% of patients with cirrhosis will develop HE. It has a negative impact on health-related quality of life due to factors such as repeated hospitalizations, frequent falls, and dependence on others for activities of daily living. Additionally, the development of HE is associated with a decrease in life expectancy, with a median survival of 2 years after development of HE (1 year if older than 65).
Discovering Ammonia
In the 1700s, Scottish physician William Saunders wrote “There seems much sympathy between the brain and the liver, and in maniacal persons, in whom there is generally a defection in the secretion of bile.” In the late 1800s, it was discovered that portosystemic shunting played a role in the development of encephalopathy when a Russian surgeon by the name of Nikolai Vladimirovich Eck studied the placement of surgical portosystemic shunts (the Eck fistula) in dogs. The urea cycle was described by Krebs in 1932 and helped drive the understanding of the role of ammonia in the neuropsychiatric syndrome complicating liver disease. In the 1940s, Raymond Adams and Joseph Foley first observed and described what they termed as “anisosterixis” for an (negative) - iso (equal) - sterixis (solidity or firmness), later shorted to asterixis. Multiplestudies in the 1950s found elevated serum ammonia levels in patients with cirrhosis and confusion when compared to healthy controls. The strongest evidence for the role of ammonia in HE came in the early 1950s when a triooftrials found that patients with cirrhosis given ammonia cation-exchange resins, nitrogenous compounds and high protein diets developed elevated ammonia levels and changes in mental status with characteristic EEG findings.
Is serum ammonia useful in the diagnosis and management of HE?
The pathophysiology and role of ammonia in hepatic encephalopathy is complex and beyond the scope of this post. It is known that ammonia a primary etiologic agent of hepatic encephalopathy, but does measuring it help us diagnose the condition? As early as 1963, Jules Stahl noted that patients with HE had elevated venous and arterial ammonia levels, but the levels did not correlate with grade of encephalopathy. In a prospective study by Ong et. al, the relationship between serum ammonia levels and severity of encephalopathy by West Haven Criteria was significant, however the correlation was incomplete (r value 0.56). In another study, both normal and elevated blood ammonia levels were found in patients with all grades of HE determined by West-Haven criteria (WHC) showing that ammonia levels cannot be relied on to necessarily rule in or rule out the diagnosis of HE (Figure 1). In patients admitted with severe HE (grade 3 or 4 per WHC), there was no difference in arterial ammonia between patients between groups, however more patients with grade 4 HE met SIRS criteria highlighting the role of inflammation in severe HE. In another study, a majority (62%) of patients with hepatic encephalopathy triggered had a normal ammonia level.
| Figure 1: Gundling F, Zelihic E, Seidl H, et al. How to diagnose hepatic encephalopathy in the emergency department. Ann Hepatol. 2013;12(1):108-114 |
Regarding monitoring response to treatment, Nicolao F, et. al showed that ammonia levels generally trended down with resolution of HE, however there were some patients who had an unchanged or even increasing ammonia level despite improvement in cognition. In another trial, ammonia levels at 6 and 24-hours following initiation of treatment for HE did not correlate with resolution of HE, suggesting low value in its use to monitor response to therapy.
When ammonia is ordered, in practice, the level does not seem to change management. In one study, providers were surveyed between ordering an ammonia level and before the results became available to determine the effect that the ammonia level would have on decision-making. It was found that in patients with a high pre-test probability of having HE, an elevated ammonia level did not impact the decision to treat, suggesting that it does not provide any benefit above clinical judgment. In another study, patients admitted with HE were assessed to determine if the presence of or level of ammonia impacted treatment during hospitalization, with no difference in the amount of lactulose given during hospitalization between patients with no ammonia level, normal ammonia level, or elevated ammonia level.
However, when an ammonia level is ordered and is within normal limits, the diagnosis of HE should be questioned. The AASLD guidelines recommend that if an ammonia levels is checked in a patient with suspected overt HE and is normal, that it calls for a diagnostic reevaluation, though they do not specifically recommend checking ammonia levels to confirm diagnosis. In contrast, the EASL guidelines give a strong recommendation to check plasma ammonia levels in patients with delirium/encephalopathy and liver disease primarily based on studies showing strong negative predictive value of normal ammonia levels.
Are ammonia levels ever useful?
While ammonia levels do not have role in diagnosis or treatment of HE, they may have a role in predicting liver-related events and in prognostication.
A recent trial developed the AMMON-OHE model to predict the risk of the development of overt encephalopathy in outpatients with cirrhosis. This model incorporated sex, presence of diabetes, albumin, creatinine, and ammonia levels, and accurately predicted the development of overt hepatic encephalopathy (OHE) in patients with cirrhosis who had not had prior episodes of OHE. Additionally, a serum ammonia level higher than the upper limit of normal was an independent predictor for the development of OHE.
Furthermore, in another study looking at ammonia levels in patients with cirrhosis, a serum ammonia level >1.4 times the upper limit of normal was an independent risk factor for future liver-related hospitalizations. This same study looked at ammonia in stable outpatients with cirrhosis, with serum ammonia level >1.4 times the upper limit of normal independently associated with an increased all-cause mortality. In this study, ammonia performed similarly to Child-Pugh or MELD score in prognosticating all-cause mortality at 1 year. In a study looking at patients admitted with acute decompensated cirrhosis, increased admission venous ammonia levels (using a level >60 µmol/L) were independently associated with decreased 30- and 90-day transplant-free survival.
Ammonia’s utility as a prognostic test may be partially explained as a marker for physiologic reserve. Increased venous ammonia may be reflective of decreased muscle mass for ammonia uptake. Sarcopenia is common in patients with cirrhosis and it is a poor prognostic factor, with sarcopenia associated with an approximately 2-fold higher risk of death. Elevated ammonia may also be reflective of impaired liver synthetic function, worsening portosystemic shunting, renal dysfunction, and intestinal dysbiosis.
Issues with ammonia measurement
Now that we have discussed the utility of ammonia levels in the diagnosis, management and prognosis of hepatic encephalopathy, lets discuss some of the issues involved with measuring ammonia. Wide variability and lability of serum ammonia levels have been reported in studies of patients with cirrhosis and healthy volunteers. Ammonia metabolism is complex and dependent on multiple organ systems (liver, muscle, kidney, brain). As a result, the precise measurement of ammonia in the blood can be challenging and is dependent on where the blood is drawn from, the way samples are handled, and how they are analyzed and reported.
When looking at plain venous samples, even when following a strict protocol, significant differences exist between testing sites, so lab-to-lab differences exist as well. The reference range for ammonia varies between centers with an upper limit of normal cited anywhere from 32 to 72 µmol/L. Thus, standardizing what constitutes an abnormal ammonia level is challenging.
One possible explanation for the variability in results may be due to the strict sample handling and processing requirements. According to labcorp:
“Patient should be fasting 12 to 14 hours to avoid lipemia, which interferes with the test. Tube must be filled completely and kept tightly stoppered at all times. Mix well. Specimen must be placed on ice immediately. Separate plasma from cells within 15 minutes of collection. Patient should not clench fist. Transfer specimen to a plastic transport tube before freezing. Avoid contamination of samples by ammonia from smoking or traffic in the laboratory or patient's room, glassware, or water.”
The reasoning behind these strict collection recommendations is largely due to the generation and release of ammonia from red blood cells even after sample collection, particularly at higher temperatures. Even at 4ºC, ammonia levels in whole blood samples are only stable for about 1 hour before they start to rise. In as few as 2 hours at room temperature, which may be how long a phlebotomy sample stays on a collection tray, ammonia levels can double (Figure 2). In plasma samples, ammonia levels remain stable for longer which is why the plasma should be separated from cells as soon as possible. The fasting component is important as well as patients with cirrhosis can have an increase in serum ammonia of 18% following ingesting of a protein-rich meal.
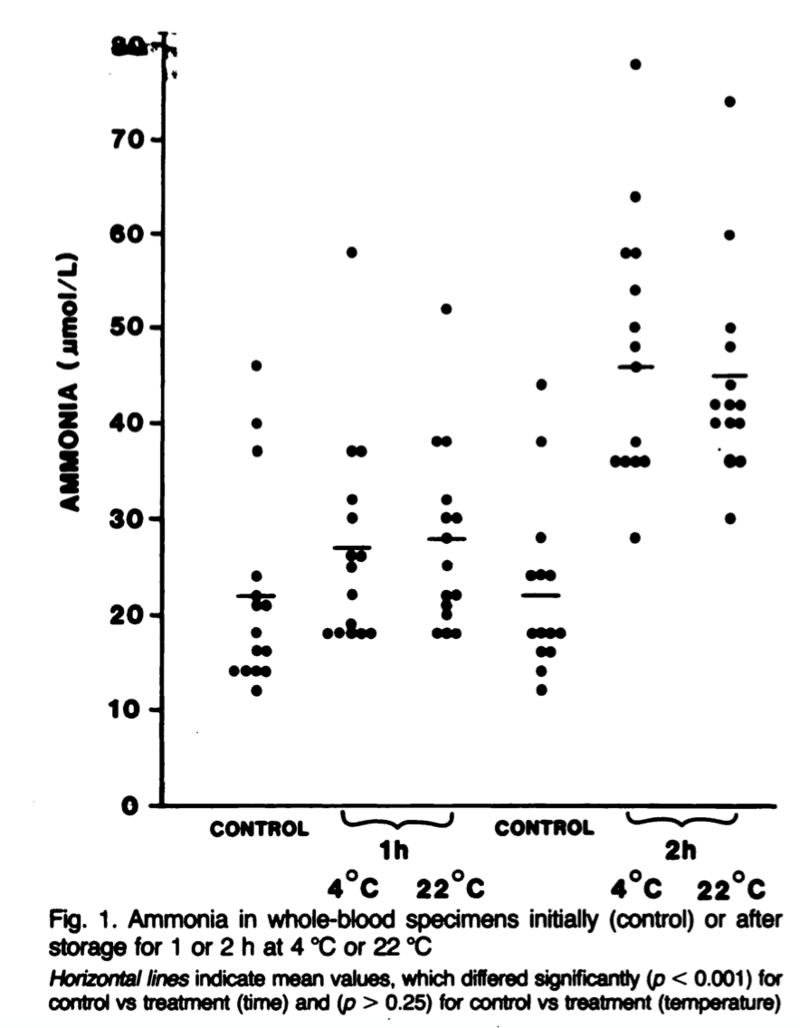 |
| Figure 2: Howanitz JH, Howanitz PJ, Skrodzki CA, Iwanski JA. Influences of specimen processing and storage conditions on results for plasma ammonia. Clin Chem. 1984;30(6):906-908. |
Additionally, the site of the blood draw can impact the ammonia level. In a 1960s study by Stahl et. Al, ammonia salts were given to patients with cirrhosis and blood samples were simultaneously collected from different arterial and venous sites (Figure 3). In general, the ammonia levels were highest in arterial blood samples and lowest in peripheral/superficial blood samples. The rationale behind this finding is that ammonia in arterial blood is taken up primarily by muscle where it undergoes extrahepatic metabolism and so the returning venous blood has lower ammonia levels. The differences in venous ammonia levels between different sites is likely in part due to the different metabolic activities of the tissues the vein is draining, however peripheral vasodilation plays an important role. Increasing peripheral vasodilation leads to an “arterialization” of the peripheral venous blood and so less ammonia is absorbed by the tissues (an important concept in cirrhosis, which is generally a vasodilatory state). This was studied by applying a heat pack to one limb to induce vasodilation, with ammonia levels drawn from that limb higher than those drawn from the opposite limb. For similar reasons, it is recommended to avoid clenched fists or use a tourniquet for collection because the increased muscle activity and venous pooling can reduce the ammonia levels.
| Figure 3: Stahl J. Studies of the Blood Ammonia in Liver Disease: Its Diagnostic, Prognostic and Therapeutic Significance. Ann Intern Med. 1963;58:1–24. |
Once the blood sample gets to the lab, the final challenge lies in the laboratory analysis and reporting of the ammonia level. Total ammonia levels are typically assayed when ordering an ammonia level, which combines the levels of both ammonia (NH3) and ammonium (NH4). However, only unionized ammonia (NH3) can freely spread through cell membranes and cross the blood brain barrier. The partial pressure of ammonia (pNH3) can be calculated using an arterial sample with a pH measurement, but whether this test is more accurate than venous or plain arterial samples isdebated.
Summary
In conclusion, the development of hepatic encephalopathy is an important transition in the natural history of cirrhosis associated with worse quality of life and shorter life expectancy. Ammonia is the primary etiologic agent, but blood ammonia levels play a limited role in the diagnosis and management of HE. Ammonia measurements do have their value in prognostication. Measuring ammonia is challenging with significant variability between levels depending on where the blood was drawn from (arterial vs. venous, peripheral vs. central), how the sample was handled, and how the test was analyzed and reported in the lab.
Take home points:
- Ammonia is required for the pathogenesis of HE but likely not sufficient alone as inflammation appears to play a large role, particularly in severe HE
- Elevated ammonia level is not diagnostic of HE, and serial measurements do not have a role in management of HE
- Elevated ammonia levels are prognostic both in the future development of OHE in patients without a history of OHE, and in regard to future hospitalizations and even mortality
- Low ammonia levels may have utility in bringing the diagnosis of HE into question
- Laboratory analysis of ammonia levels is imprecise due to the complexities of ammonia metabolism which results in changes to ammonia levels depending on where the sample is collected from, how it is handled, and how it is reported


