Why Do We Care About Portomesenteric Venous Thrombosis (PMVT) in Cirrhosis?
Background, Pathophysiology and Epidemiology
Portomesenteric venous thrombosis (PMVT) is a frequently-observed finding in patients with cirrhosis. The most common type of PMVT is portal vein thrombosis (PVT). The identification of a PMVT may be the result of a workup for hepatic decompensation or an incidental finding on imaging obtained for another indication. While PMVT is more common in people with cirrhosis, it can also be found in noncirrhotic liver disease and other predisposing factors. This post will focus primarily on PVMT in the setting of cirrhosis.
The unique vascular architecture of the liver lends itself to its necessary functions, while also creating opportunity for thrombi to form. In contrast to most organs, the liver’s blood supply is drawn from both arterial and venous circulation. The arterial flow, which is made up of downstream arterioles and capillaries from the hepatic artery is the primary source of blood for the biliary tree (e.g. cholangiocytes). The venous blood supply is derived from the portal vein, which is a confluence of mesenteric tributaries such as the splenic, superior mesenteric, and inferior mesenteric veins (Figure 1). The portal vein is the primary blood supply to the hepatic parenchyma (e.g. hepatocytes).
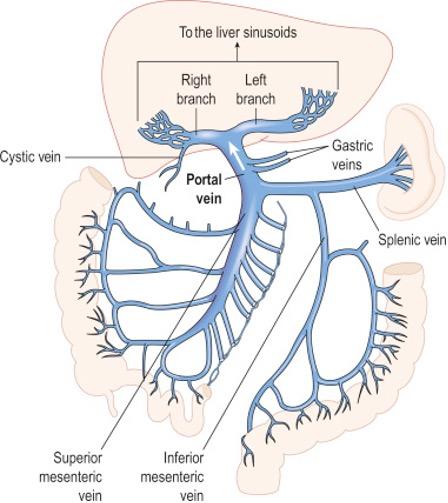
Figure 1: Portal venous system with confluence in main portal vein (Source: Carlson BM, The Human Body, 2019).
Due to its baseline low-flow state, the portomesenteric venous system is highly susceptible to the development of thrombosis. This is particularly relevant to patients with liver disease as they have a baseline coagulopathy (see our “Back to Basics” on this topic) and portal hypertension that creates further resistance to flow. Additionally, ascites and associated gut edema result in constant bacterial translocation. This, in turn, causes endothelial damage and local inflammation that further drives clot formation. While the prevalence is unknown, one study found that approximately 10% of patients with cirrhosis have PMVT, with hepatocellular carcinoma associated with increased risk (Table 1). The prompt identification of PMVT is crucial, as it has important implications for management and prognosis.
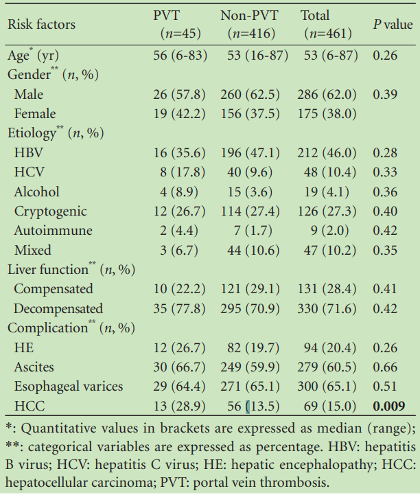
Table 1: Prevalence of PMVT in patients with cirrhosis and etiologies of liver disease in patients with PMVT.
Diagnosis
Initial Evaluation
Clinical findings that should prompt evaluation for PMVT in patients with liver disease include unexplained abdominal pain, as well as new or recurrence of prior hepatic decompensation. Yet, PMVT is often asymptomatic.
When a new PMVT is suspected, it is also important to evaluate for other contributing risk factors—including history of clotting disorders, malignancy, recent surgeries, abdominal infections, medication exposures (oral contraceptives, chemotherapy). For individuals with a diagnosis of cirrhosis and no other clear risk factors for PMVT, the AASLD states that it may not be necessary to pursue further evaluation for other underlying etiologies. In the absence of suggestive history for other contributing factors, testing for underlying coagulopathy in patients with cirrhosis may not be worthwhile given its relatively low prevalence (Factor V Leiden: 6.7%, Prothrombin G20102A mutation: 5.4%).
Additionally, the carriers of these mutations with concomitant cirrhosis have not been shown to be at increased risk. In one comprehensive Spanish cohort study, of the 263 patients for which Factor V Leiden and G20102A mutation analysis were available, none with positive mutations developed PMVT. Thus, evaluation for these mutations are of minimal clinical utility in risk-stratifying PMVT development patients with cirrhosis and are unlikely to affect management.
Imaging
When PMVT is suspected, abdominal ultrasound (US) with Doppler is the preferred initial modality. This is due to its low cost and the ability to assess to vasculature without the need for radiation or contrast exposure. Ultrasonographic features consistent with PMVT include anechoic material in the portal vein and absence of venous blood flow on Doppler. The diagnostic sensitivity of US for diagnosis of PVT is estimated to be between 66% and 100%. However, US has several limitations in the evaluation of PMVT. It may be inadequate to confirm the presence of a thrombus due to poor acoustic windows or interference from overlying bowel gas. US is also unable to characterize the full portomesenteric venous system, limiting the ability to evaluate total clot burden.
Consequently, even when US findings are suspicious for PMVT, cross-sectional imaging (CT or MRI) with intravenous contrast is needed to confirm the diagnosis. Cross-sectional imaging allows complete evaluation of the portomesenteric venous system as well as identification of porto-systemic collaterals that have implications in procedural interventions and may assist in determining chronicity. Another key benefit to cross-sectional imaging is that it is more sensitive for diagnosis of neoplastic invasion of the portal vein, which has significant management implications. MRI and CT are comparable with respect to evaluation of thrombus, but MRI may offer additional sensitivity in identification of underlying hepatocellular carcinoma.
Classification
Several PMVT classification schemes have been proposed with grouping based on anatomic location, degree of occlusion, duration, and presence of associated tumor. However, there remains no widely-accepted system and nomenclature remains heterogenous. Ultimately, the characterization of PMVT is most relevant with respect to its prognostic and treatment implications (Table 2).
Descriptor |
Definition |
|---|---|
| Time Course | |
| Recent | PVT presumed to be present for fewer than 6 months |
| Chronic | PVT present or persistent for more than six months |
| Percent Occlusion of Main PV | |
| Completely occlusive | No persistent lumen |
| Partially occlusive | Clot obstructing over 50% of original vessel lumen |
| Minimally occlusive | Clot obstructing less than 50% of original vessel lumen |
| Cavernous transformation | Gross portoportal collaterals without original PV seen |
| Response to Treatment or Interval Change | |
| Progressive | Thrombus increases in size or progresses to more complete occlusion |
| Stable | No appreciable change in size or occlusion |
| Regressive | Thrombus decreases in size or degree of occlusion |
Table 2: AASLD guidelines on nomenclature for classification of PMVT.
Perhaps the most important determination is whether the thrombus is associated with a malignant tumor such as HCC or metastatic colorectal cancer. With respect to the clot itself, this has critical therapeutic implicants as treatment of tumor thrombus does not generally impact prognosis. Additionally, if the findings reflect a new cancer diagnosis, there are numerous other diagnostic and therapeutic considerations that require multi-disciplinary discussion.
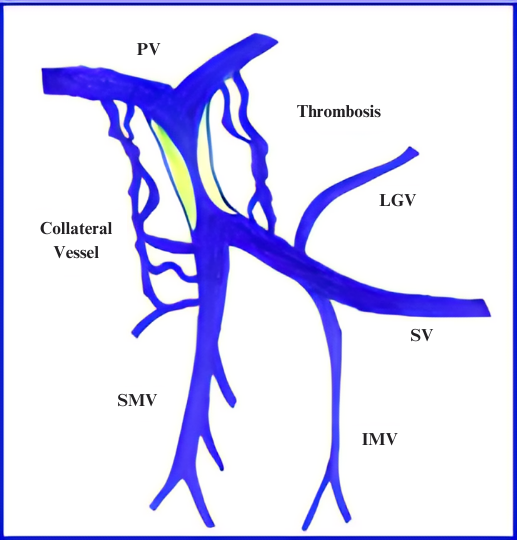
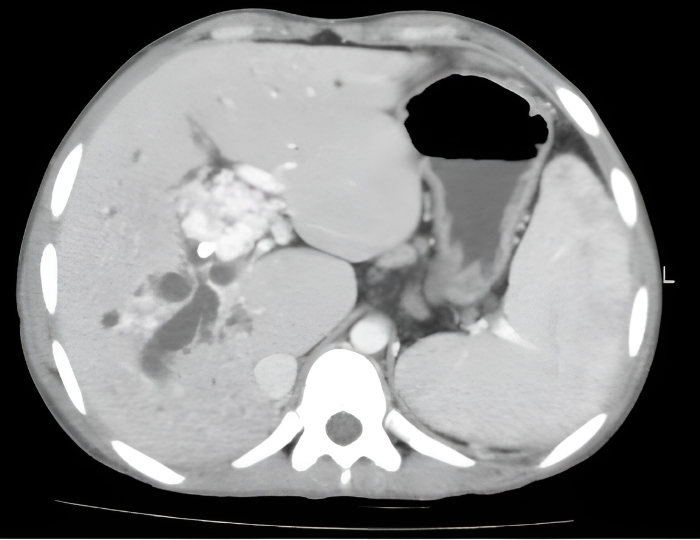
Figure 2: (A) Depiction of portal venous system with PVT. Note associated collateral vessel dilation (cavernous transformation) in response to sluggish flow in the PV. (B) Axial computed tomographic appearance of portal cavernous transformation with dilated vessels that enhance in portal venous phase.
Another important feature includes the degree of occlusion (e.g. minimal vs. partial vs. complete). In particular, complete occlusion is associated with more frequent cavernous transformation and lower likelihood of regressing with anticoagulation. Finally, a key consideration, especially as it relates to transplant, is the clot burden. Involvement of the superior mesenteric vein can complicate surgical planning for transplant candidates and, in rare cases, lead to intestinal ischemia. It is important to note, however, that the congestive ischemia from PSVT is less common in patients with cirrhosis.
Management
Decision-making regarding the management of PMVT is highly patient-specific and often requires multidisciplinary discussion. The ultimate goal of treatment of PVMT is to decrease symptoms (if present), prevent complications related to chronic venous occlusion, and maximize probability of successful liver transplant in otherwise suitable candidates. There is limited data to guide decision-making, but some patient-specific factors to be considered are:
- Overall clinical and functional status
- Symptoms directly attributable to thrombus
- Risks of anticoagulation or procedural intervention (including sedation)
- Presence of other comorbidities associated with the thrombus (e.g. infection and ischemia)
- Transplant candidacy
The AASLD provides a general treatment algorithm but there is little objective data to support the treatment decisions recommended (Figure 3).
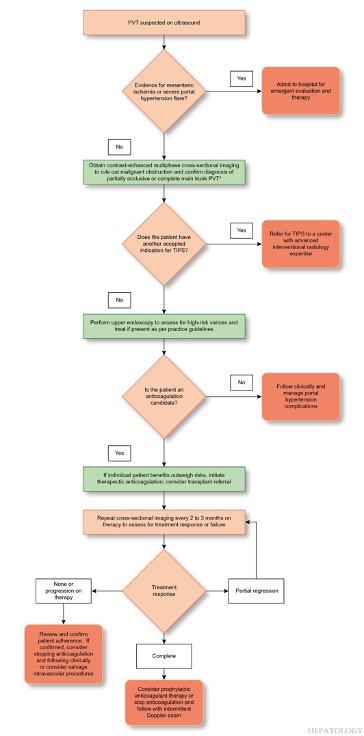
Figure 3: Recommended management algorithm for PVT by the AASLD.
Medical Therapy
With respect to treatment modalities, individuals with cirrhosis PMVT may require medical therapy, procedural intervention, or a combination. Anticoagulation with warfarin or heparin (low-molecular weight or unfractionated) and is the most well-studied.
However, given the challenges regarding therapeutic monitoring in patients with cirrhosis and the challenges of self-injecting, direct oral anticoagulants (DOACs) are becoming mainstay therapies. New data suggests a reasonable safety profile with one Japanese study demonstrating superior resolution of PVT with a DOAC compared to warfarin (77% vs. 29%). In a recent systematic review, combined data from 2,469 patients with cirrhosis on DOACs demonstrated an overall risk of bleeding that was lower (OR = 0.71) than traditional anticoagulants (Figure 4).
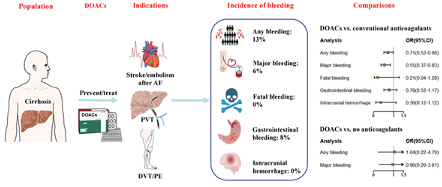
Figure 4: Incidence of bleeding events in patients with cirrhosis on DOACs.
In patients with cirrhosis, data regarding treatment of asymptomatic PMVT remains mixed. This is, in part, because prior studies have primarily evaluated patients in the context of liver transplant. For patients with partially or completely occlusive thrombus in PV, anticoagulation should be considered up-front to prevent portal hypertensive complications and extension. However, in patients with recent (<6 months), minimally-occlusive (<50% of lumen) in main PV, surveillance with ultrasound is reasonable to observe for resolution.
If progression is seen, then treatment would be considered. For people with cavernous transformation of the PV, there is no clear benefit to medical therapy. Additionally, presence of splenic vein thrombosis has also been associated with treatment futility (Figure 5). It is important to note that this has been largely extrapolated from patients with noncirrhotic portal hypertension.
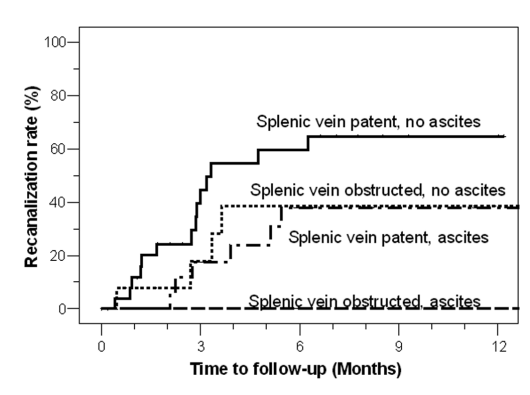
Figure 5: Association of concomitant splenic vein thrombosis +/- ascites and recanalization rate of portal vein.
One area of controversy pertaining to therapy initiation is the role of screening for esophageal varices (EV) at time of PMVT diagnosis. Though there is data to support that EVL ligation can be safely performed on anticoagulation, there remains significant practice heterogeneity with respect to timing of screening. The AASLD recommends immediate initiation of anticoagulation with subsequent EV screening to avoid delays in therapy. However, it may be reasonable to defer initiation for patients who can receive urgent endoscopic evaluation.
Procedural Intervention
Procedural therapies for PMVT include catheter-directed thrombolysis and direct thrombectomy with or without subsequent TIPS placement. Early data for these modalities were mixed, and they have previously been associated with significant morbidity and mortality.
In one small Dutch study (n=12), six patients (50%) had severe bleeding complications and with 2 subsequently experiencing death. However, a recent study in patients with acute PVT and intestinal ischemia suggests that a stepwise approach with trial of thrombolytics followed by TIPS thrombectomy as salvage is reasonable. In this cohort of 22 patients, 14 (55%) failed medical therapy and subsequently underwent TIPS thrombectomy with successful recanalization in 11 patients. In this group, overall success of recanalization was 86% with only 2 patients experiencing major complications. It is important to note that only 2 patients in this group had underlying cirrhosis.
A circumstance in which procedural intervention should be considered upfront is chronic PMVT with cavernous transformation in patients who remain otherwise suitable transplant candidates. In this situation, recanalization of the PV provides added benefit of possible physiologic anastomosis between the native and donor PV. A meta-analysis of studies representing a total of 399 patients found an overall success rate of TIPS thrombectomy to be 95%, but with cavernous transformation of being a predictor of technical failure. Following procedural intervention, the need for and duration of systemic anticoagulation remains unclear. Little is known about the long-term risk of recurrent PMVT in patients who have undergone these therapies.
Key Points:
- Portomesenteric venous thrombosis (PMVT) is a reasonably common complication in patients with cirrhosis.
- Identification of PMVT may be made in the evaluation of symptoms or hepatic decompensation, but is often incidental.
- Critical factors in determining the treatment plan include presence of malignancy and duration of clot.
- The decision to treat PMVT must also account for patient-specific factors such as transplant candidacy.
- Medical therapy with warfarin, heparin derivatives, and direct oral anticoagulants is generally the preferred strategy.
- For transplant candidates with occlusive PV thrombus and cavernous transformation, procedural intervention to achieve recanalization may be beneficial to allow physiologic anastomosis between donor and recipient PV.
