Why do we use early TIPS for the management of variceal bleeding?
In this series, we will talk about the natural history of variceal bleeding, current standard of care, patients defined as high risk for treatment failure, role of early TIPS and its limitations in the setting of variceal bleeding.
What is the natural history of variceal bleeding?
Among decompensating events in cirrhosis, variceal bleeding has had the most advances in terms of management over the past 4 decades. Here is a study by Graham et al from 1981, which showed the very high mortality of variceal bleeding. As you can see in the graph, the majority of re-bleeding events and mortality happened in the first 6 weeks, with most events occurring in the first 5 days. With only surgical shunts for a select few and Blakemore as therapeutic options, these data give a stark view of the natural history of variceal hemorrhage.



With the current standard of care, there has been a 4-fold improvement in survival (see the survival graphs below comparing 1980s with 2020).
What is the standard of care?
The current standard of care based on AASLD and Baveno includes the following:
Initial steps prior to EGD:
- Resuscitation with restrictive transfusion strategy
- targeting 7 g/dL is better than 9 (in patients without shock)
- Vasoactive agents (octreotide, somatostatin, terlipressin)
- As early as possible/prior to endoscopy → continue for 2-5 days→ transition to NSBBs
- Short term antibiotics (ceftriaxone 1g/d, max 7 days)
Endoscopic evaluation
- Early therapeutic endoscopy (< 12 hrs) → Endoscopic band ligation (EBL)
Next steps after control of bleeding:
- If the patient responds to treatment and no further bleeding → transition to secondary PPX: NSBBs + serial banding
- Treatment failure → salvage or rescue TIPS
Baveno and AASLD recommend mortality outcome to be defined as death from any cause in the first 6 weeks after a bleeding event. Mortality in the setting of variceal bleeding is not always from bleeding itself, but given majority of the events in the acute phase are related to the episode of bleeding, the consensus is to count all deaths in the first 6 weeks as VB related mortality. Treatment failure is defined as a failure to control bleeding or re-bleeding within the first 5 days.
The above algorithm of treatment, which is the current standard of care, has significantly improved survival in acute variceal bleeding. In multiple real world retrospective studies, the 6 week mortality ranges from 10-20%. Most of the deaths happen in cases with treatment failure or in those requiring rescue TIPS, and mortality in this specific group ranges between 30-66% based on different studies. It is also important to note that despite good evidence for the improved survival with the current standard of care, adherence to best practice has room for improvement. For example a systematic review of cohort studies of variceal bleeding outcomes from 1990 to 2016 found that only half the patients received prophylactic antibiotics.
Now that we know patients who have treatment failure even after receiving rescue TIPS are at the highest risk for mortality, why not place the TIPS before treatment failure happens and things start to go downhill! Hold this thought, we’ll come back to this later after we learn about TIPS!
What is a TIPS?
Transjugular intrahepatic portosystemic shunt (TIPS) is a very effective treatment for complications of portal hypertension such as variceal bleeding and refractory ascites. TIPS provides a pathway between the hepatic vein and the portal vein, relieving the high portal pressures by bypassing the cirrhotic liver.
The first use of TIPS in humans for variceal bleeding dates back to 1982, where Colapinto et al described a patient who presented with massive variceal bleeding not controlled with a Blakemore tube, who was not a surgical candidate. He was therefore referred for transhepatic embolization of varices. During the procedure, they were able to a create a shunt between the hepatic and portal veins. The patient did not survive; however the autopsy showed a patent channel between the hepatic vein and portal system, without any tear in the vessels, or hemorrhage in the liver. This paved the path for future successful TIPS placement.

How did we use TIPS early on?
First, we must define the patients who are at risk for treatment failure (inability to control bleeding or re-bleeding within the first 5 days despite treatment). Here is a study by Miotinho et al that shows that the majority of treatment failure happens in patients with HVPG > 20. They describe 65 patients with AVH who underwent HVPG measurement within 20 hours of admission. The study is from 1990s, so they were not getting the current standard of care (patients were receiving medical therapy with somatostatin infusion OR endoscopic sclerotherapy as the first line of treatment). They defined “poor evolution” as failure to control bleeding or early variceal rebleeding (within one week). As you can see the majority of cases of “poor evolution” happened in patients with HVPG >20.


What about randomized trials of early TIPS?
The study by Monescillo et al was the first study to look at early TIPS in patients at high risk of treatment failure. This was a single center study in Spain from 1997 – 2000. The study group enrolled 116 patients and measured HVPG within 24 hours of hospital admission. They randomized the subset of patients with HVPG>=20 (defined as high risk group (HR)), to early TIPS (bare metal stent) vs SoC (standard of care). It is important to note that the SoC in this study was norfloxacin + somatostatin in ED only + sclerotherapy and then transition to NSBB or EBL, with a transfusion goal >9, so very different than the current standard of care. As you can see in the graph below, the high-risk patients in the early TIPS group did much better!

However, there are 2 major factors to consider about the results of this study. Firstly, performing HVPG measurements in all patients presenting with variceal bleeding is not feasible. Secondly, one could argue that maybe with the new SoC things would be different.
Garcia-Pagan et al performed an RCT to address the question. This was a multicenter study including 9 European centers from 2004 – 2007. They enrolled 63 patients with clinical criteria that was considered to be predictive of treatment failure. This population was defined as Child C with CPT score of < 14 and Child B with active bleeding. Patients were randomized to SoC (ppx Abx + EBL or sclerotherapy + vasoactive drugs), vs TIPS (covered stent) within 72 hours. One year survival was 86% for TIPS vs 61% for SoC.
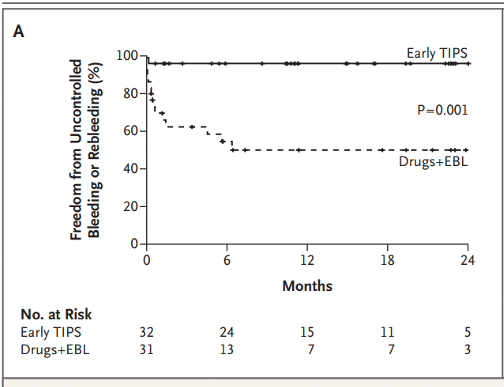

Then there was the largest study by Lv et al. This was a single center study conducted in China. The study group enrolled 132 patients with Child B and Child C < 14 presenting with active variceal bleeding. The primary outcome was transplant-free survival, assessed at 1 year. 33% in the control group versus 18% in the TIPS group died. This is the only trial that included all Child B patients.

The most recent study is by Dunne et al. This study, however, did not show similar results to prior studies. This was a study from 2 centers in UK. They enrolled 58 patients with Child B and active bleeding or Child C < 14 presenting with variceal bleeding. Patients were randomized to early TIPS vs SoC. The primary outcome was 1-year survival; 24% in the control group versus 21% in the TIPS group died, which was not statistically different. However, the study was stopped early due to slow recruitment and was underpowered for the primary outcome. Additionally, from the 29 patients randomized to early-TIPS, 6 did not receive TIPS, and 10 received TIPS outside the 72-hour window.

How do we re-define the high risk population for early TIPS?
There is a debate as to whether we need to re-define the high-risk population that could benefit from preemptive TIPS. HVPG > 20 has been a great predictor but is not a practical approach. Child B with active bleeding and Child C patients who are not too sick (<14), have been shown in to benefit from early TIPS.
There is only one trial that shows benefit in ALL Child B patients. However, it is unclear if these results would be generalizable, as the etiology of cirrhosis was hepatitis B in 74% of the patients in this study. Also, these patients had very high portal pressure gradients in comparison to other trials, so the benefit was more pronounced (see the table below for comparison).
There might be newer or better markers such as CLIF-C score or MELD.
What are the limitations of early TIPS?
There are limitations to the early TIPS approach. Before implementing this in clinical practice, first, it is important to note that these are highly selected populations. Here is a summary of the exclusion criteria from the Garcia-Pagan study. The exclusion in the other trials is very similar to this list.
- Age above 75 years
- Pregnancy
- Hepatocellular carcinoma outside Milan criteria
- Creatinine > 3
- Child–Pugh > 13
- Previous NSBB + endoscopic treatment to prevent rebleeding
- Previous portosystemic shunt or TIPS
- Bleeding from isolated gastric or ectopic varices
- Total portal vein thrombosis
- Heart failure
It also should be noted that even in a clinical trial setting, placement of TIPS can have logistical challenges, for example in the Dunne trial less than half of the patients randomized to the intervention group got early TIPS within the 72 hour window. Although most trials defined early TIPS as within 72 hours, it is possible that we might see the benefit even when placed beyond this timeframe, as long as it happens prior to re-bleeding; as such, some would prefer the terms “pre-emptive” or “prophylactic”.
And lastly the potential complications of TIPS, most importantly HE should be considered. While the first 3 trials did not show increase in occurrence of HE in the TIPS vs the SoC groups, the Dunne et al trial showed a significant difference between the 2 groups (12/29 in TIPS vs 5/29 in SOC developed HE, p=o.o5). This is challenging; while the decision for placement of rescue TIPS is more straight-forward given you are saving a life from variceal bleeding and future risk of HE is going to be less important, with early TIPS if placed in patients with low risk of re-bleeding or treatment failure, you will be exposing them to the risks of the procedure and potential future HE without added benefits.



