Specific and non-specific histopathological changes of porto-sinusoidal vascular disease
Brief Case Presentation
A 24-year-old lady with elevated but fluctuating serum alkaline phosphatase levels was referred to clinic. She was asymptomatic, had no recent episodes of jaundice, fever, or chills, and denied alcohol, tobacco, recent medication changes, or drug consumption.
Four years prior, she had an episode of bilateral pneumonia and pancytopenia on a blood smear test. Further hematological workup led to the diagnosis of common variable immunodeficiency (CVID) associated with Evans syndrome (autoimmune hemolytic anemia and immune thrombocytopenia)1,2. Since then, she has been treated with intravenous immunoglobulins.
The current laboratory analysis showed mildly elevated liver aminotransferase and moderately elevated alkaline phosphatase levels, as shown in the table below.
| Lab Test | Result |
| AST |
64 IU/L (range: 15-41 IU/L) |
| ALT |
67 IU/L (range: 14-54 IU/L) |
|
Alkaline Phosphatase |
314 IU/L (range: 38-126 IU/L) |
| Total Bilirubin |
0.4 mg/dL (range: 0.3-1.5 mg/dL) |
Serology workup for autoimmune antibodies (ANA, AMA, LKM, SMA) and hepatitis A, B, and C was negative.
Abdominal ultrasound revealed a non-cirrhotic liver, but Fibroscan revealed increased liver stiffness (8.7 kPa; normal range: 2-7 kPa), suggesting fibrosis. Splenomegaly (splenic craniocaudal length of 15 cm) has also been reported.
A liver biopsy was performed to confirm fibrosis staging and identify the cause of elevated serum alkaline phosphatase levels.
***
Microscopic Findings
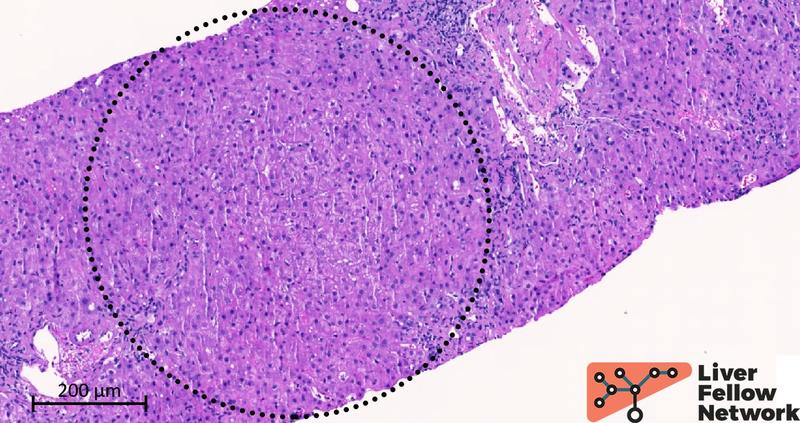
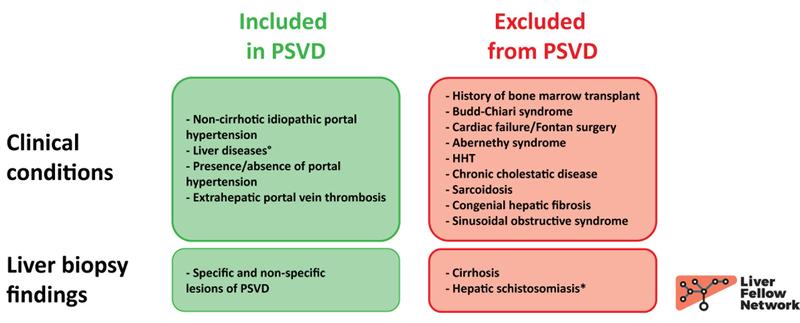
Two cores of hepatic parenchyma measuring approximately 2.5 cm in total length with about 20 portal tracts are available for examination. Overall, the liver architecture is mildly disrupted, with an irregular distribution of portal tracts (Figure 1) and a vague nodular appearance (Figure 2).
Figure 1. Liver biopsy, low-power image of one of the two cores available for analysis. Black circles outline the portal tracts.
Figure 2. Medium-power details of the lobule. The dotted circle outlines a nodular area of the liver parenchyma. In hematoxylin and eosin staining, nodularity is subtle and challenging to identify. No apparent fibrosis delineates the nodular area.
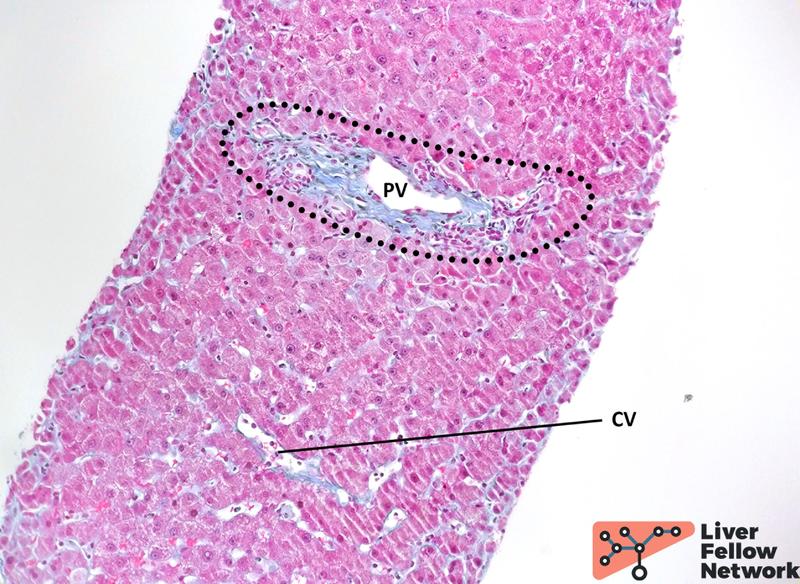
Medium-power analysis of hepatic parenchyma reveals several nodular areas. These nodules are emphasized by reticulin staining and present central thick/hypertrophic and peripheral thin/hypotrophic hepatic plates (Figure 3) but are not delimited by fibrosis: Trichrome stain highlights mild portal/periportal fibrosis, with no signs of bridging fibrosis (Figure 4).
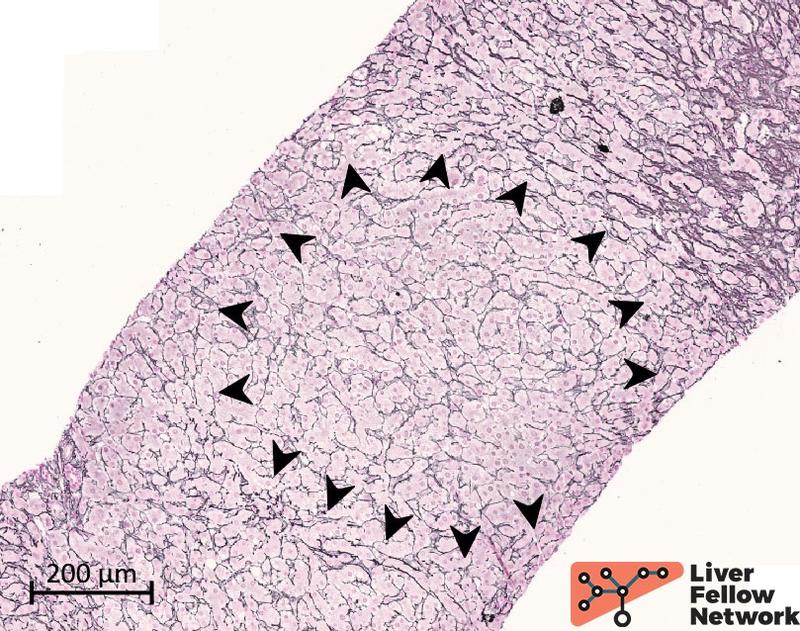
Figure 3. Reticulin stain highlights parenchyma nodules. The parenchyma nodularity is evident with the reticulin stain and is related to the hepatic plate thickness: a peripheral rim of thin/hypoplastic hepatocytes (black arrowhead) borders a central area with thicker/hyperplastic hepatocytes.
Figure 4. Trichrome staining reveals minimal fibrosis. Extracellular collagen deposits (blue-stained) are minimally increased in the portal tract (dotted area), with no expansion in the periportal area. No significant subsinusoidal or pericentral fibrosis is noted. PV: portal vein; CV: central vein.
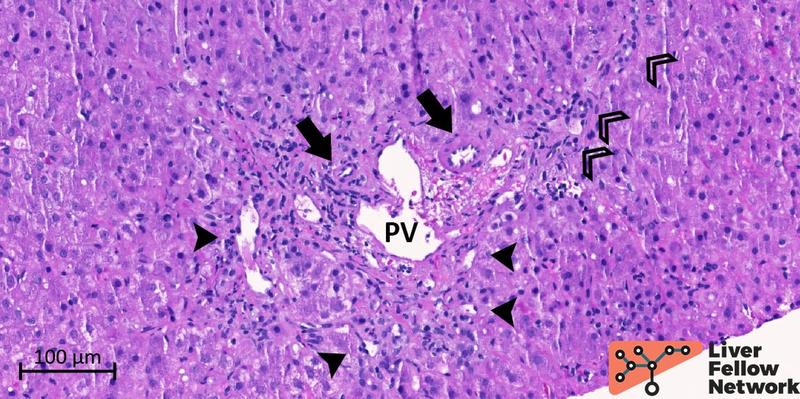
Examination of the portal/periportal areas shows portal veins with thick walls, abnormal profiles, and narrowed lumens. At least two portal tracts demonstrate portal veins with obliterated lumen (Figures 5 and 6).
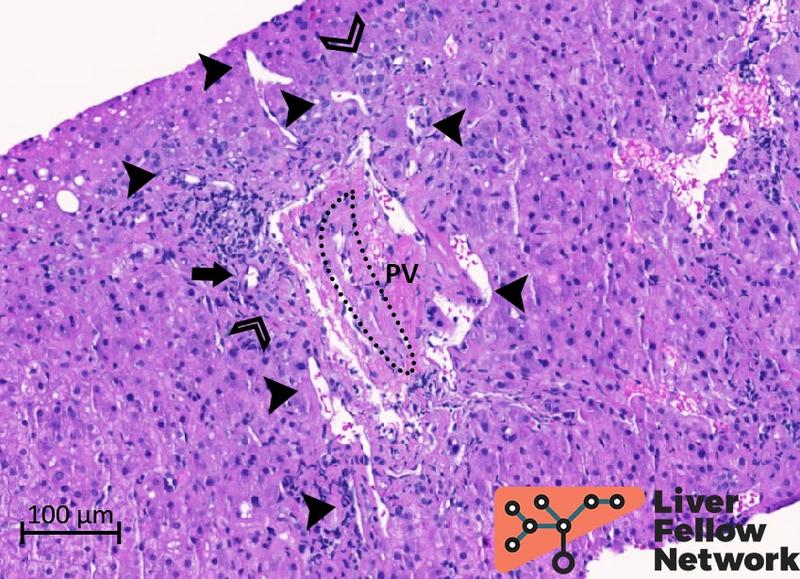
Figure 5. Medium-power details of a portal tract with obliterated portal vein. The portal vein (PV; dotted oval) lumen is almost completely obliterated. Several abnormal vessels (black arrowheads) are present in the periportal area. Mild mononuclear inflammation, bile duct and minimal ductular reaction (empty arrowheads), and hepatic artery (black arrow) are also noted.
Figure 6. Medium-power details of a portal tract with severe portal vein stenosis. In this portal tract, the portal vein cannot be clearly identified and is replaced by an area of dense fibrosis (dotted oval) with several abnormal portal and periportal vessels (black arrowheads). The bile duct (empty arrowhead) and the hepatic artery (black arrow) are normally represented.
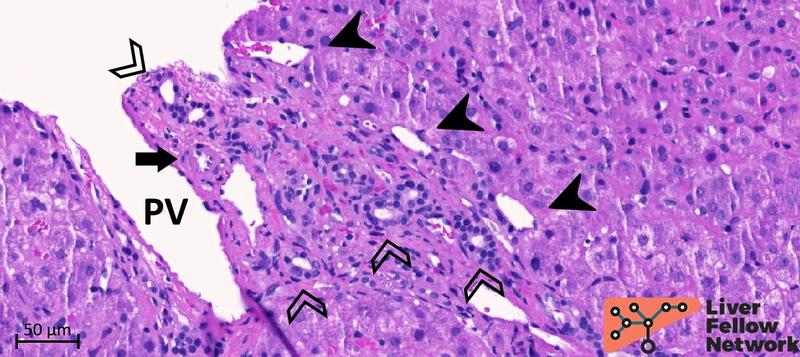
Portal tracts with near-normal portal veins show several periportal abnormal vessels with minimal mononuclear portal inflammation and mild ductular reaction (Figures 7 and 8).
Figure 7. Medium-power of a portal tract with near-normal portal vein. The portal vein (PV) shows mildly increased wall thickness and a near-normal lumen profile. However, several aberrant periportal vessels (black arrowheads) are observed. The bile duct and mild ductular reaction (empty arrowheads) and hepatic artery (black arrow) are noted.
Figure 8. Medium-power of a portal tract with near-normal portal vein. Similar to Figure 7, the portal vein (PV) has a patent lumen, but the profile is irregular and shows branch herniation toward the periportal hepatocytes. Portal and periportal abnormal vessels (black arrowheads) are also identified. The hepatic artery (black arrow) and the bile duct (empty arrowhead) are regularly represented.
No morphological signs of bile duct injury or ductopenia are observed.
In the lobule, mild mixed predominantly small droplet macrovesicular steatosis involving less than 5% of hepatocytes is noted but without evident signs of steatohepatitis (lobular inflammation, hepatocyte ballooning degeneration, Mallory-Denk bodies, megamitochondria, or apoptotic bodies). Minimal sinusoidal congestion is observed in the pericentral area (Figure 9).
Figure 9. Medium-power details of the sinusoidal congestion in the pericentral area. The central vein (CV) is well represented, and only minimal congestion of the pericentral sinusoids (black arrowhead) is noted.
Based on the clinical history (elevated alkaline phosphatase, splenomegaly, and CVID with Evans syndrome) and the histopathological changes observed (portal vein stenosis, nodular regenerative hyperplasia, absence of severe fibrosis/cirrhosis, and no signs of bile duct injury), what is your diagnosis in this young female patient?
This is a case of porto-sinusoidal vascular disease (PSVD).
***
PSVD: recommendations for histopathology interpretation and diagnosis.
Definition and diagnostic criteria
This edition of the Pathology Pearls reports a case with typical clinical and histopathological features of PSVD.
PSVD is the new nomenclature proposed by the Vascular Liver Disease Interest Group to identify a complex and multifaceted disease affecting intrahepatic microvascular structures3. This term encompasses previous definitions, including "idiopathic non-cirrhotic portal hypertension", and expands the spectrum of conditions classified as PSVD now including patients without clinically evident portal hypertension (Figure 10).
Figure 10. Inclusion and exclusion criteria of PSVD according to the new proposal.
°: it indicates known causes of liver disease development (e.g., alcohol assumption, metabolic syndrome, hepatotropic virus infection);
*: hepatic schistosomiasis needs to be confirmed on liver biopsy and should not rely on positive serology only.
Adapted from:
- De Gottardi A et al. Lancet Gastroenterol Hepatol. 2019 May;4(5):399-411 3
- De Gottardi A et al. J Hepatol. 2022 Oct;77(4):1124-1135 4
Liver biopsy is fundamental for a definitive diagnosis, and along with the new nomenclature, specific and non-specific histologic lesions have been proposed as part of the histopathological spectrum of the disease3,4.
The following paragraphs detail the main characteristics of these histological lesions, potential diagnostic pitfalls, and useful ancillary stains.
***
Specific histology lesions of PSVD: portal vein stenosis, nodular regenerative hyperplasia, and incomplete septal fibrosis
1. Portal vein stenosis refers to partial or complete obliteration that can occur in portal vein branches with or without thickening of the vein wall. This term has recently been proposed as a better alternative to obliterative portal venopathy and portal phebosclerosis to represent the whole spectrum of histopathological changes that can affect the portal vein5.
Indeed, portal vein stenosis with wall fibrotic thickening and significant lumen narrowing was evident in our case, but it could be subtle and heterogeneously distributed. Comparing the morphology and dimension of the portal vein with those of the bile duct or hepatic artery helps identify minor changes in portal vein stenosis (the portal vein is three to four times larger than the bile duct/hepatic artery). A potential diagnostic pitfall can be misinterpreting a small portal tract with a completely obliterated portal vein as a portal "dyad" (a portal tract with only a bile duct and hepatic artery, with no portal vein branch). This problem is especially relevant because core biopsies mostly sample the peripheral liver parenchyma, where portal dyads are more frequently observed6. In this scenario, an area of dense fibrosis in the portal tract favors severe portal vein stenosis with complete lumen obliteration, as portal dyads are usually small with a scant extracellular matrix.
2. Nodular regenerative hyperplasia (NRH) is the formation of hepatic parenchymal nodules without increased fibrosis7. NRH originates from a direct injury to sinusoidal endothelial cells or intrahepatic microvascular structures (small branches of portal veins and hepatic arteries) but can also be secondary to suboptimal pre-hepatic flow or post-hepatic venous drainage. Under these conditions, normal intrahepatic blood flow is altered, resulting in areas of parenchyma with decreased perfusion and, consequently, hypotrophic/atrophic hepatocytes. Compensatory hypertrophy in hepatocytes exposed to normal or increased blood flow leads to a nodular appearance of the liver parenchyma.
NRH nodules are typically small (range: 1-3 mm) but can be multiple and diffuse, as observed in our case. Sometimes, a rim of dilated sinusoids delimitates the nodule's periphery and facilitates NRH identification. However, NRH changes can be subtle on hematoxylin and eosin but are more evident with reticulin staining (see Diagnosing PSVD: ancillary stain and potential pitfalls paragraph).
Several hepatic (cholangiopathies, Budd-Chiari syndrome, congestive hepatopathy) and extrahepatic (rheumatoid arthritis, systemic lupus erythematosus, chronic lymphocytic leukemia, Hodgkin lymphoma) diseases can present with NRH changes. Additionally, NRH can be induced by hepatotoxic drugs, as observed in liver transplant patients treated with azathioprine.
Clinically, NRH alone can lead to a (mild) increase in serum alkaline phosphatase or gamma-glutamyl transpeptidase levels without evidence of cholangiopathy. In our case, a complete histochemical and immunohistochemical biliary workup has been performed due to the increased serum alkaline phosphatase levels, but it resulted negative for cholangiopathy.
3. Incomplete septal fibrosis is defined as the presence of thin and incomplete fibrotic bundles that delineate partial nodules in the liver parenchyma8. Interpreting fibrosis septa as incomplete and distinguishing them from regressive cirrhosis is problematic and should be carefully performed on extensive liver parenchyma. This distinction is particularly relevant, as cirrhosis (i.e., diffuse disruption of normal liver architecture characterized by fibrous subdivision of the liver parenchyma into structurally abnormal nodules) is currently an exclusion criterion for PSVD diagnosis. This diagnostic conundrum can be solved by thoroughly reviewing the patient's clinical history to exclude causes of regressive cirrhosis (i.e., history of chronic liver disease under treatment) and prevent diagnostic misinterpretations.
In our case, there was no significant fibrosis (Figure 4); however, we observed parenchymal steatosis and sinusoidal congestion (Figure 9), which may have contributed to the reported increased liver stiffness value. Additionally, PSVD has been associated with increased liver stiffness values (mean: 8.4 kPa; median: 7.8-8 kPa).
***
PSVD: ancillary stains
Histopathology changes in PSVD can be mild and heterogeneously distributed. The following is a list of histochemical and immunohistochemical stains that can facilitate the identification of these lesions and the diagnosis of PSVD.
Reticulin staining is the most effective and appropriate stain for identifying NRH. Reticulin fibers (type III collagen) in the space of Disse are highlighted by a black stain over a pale pink background, thus emphasizing the thickness of the hepatocyte plates and making evident the nodular arrangement of hypotrophic and hypertrophic hepatocytes (Figure 11).
Figure 11. Different grades of NRH on reticulin staining. NRH can range from minimal and subtle (A) to evident (B) and severe (C). Dotted circles highlight the nodules and black arrowheads indicate hypotrophic hepatocytes and collapsed reticulin fibers at the periphery of NRH nodules.
Immunohistochemical staining for cytokeratin 7 can also help identify NRH changes, as hypotrophic hepatocytes at the periphery of an NRH nodule can acquire a biliary phenotype (biliary metaplasia) secondary to reduced blood flow9. However, attention should be paid to the staining pattern: biliary metaplasia is a non-specific response of hepatocytes to injury and can be observed in cholangiopathies (periportal hepatocytes) and following ischemic injury (pericentral hepatocytes). Therefore, biliary metaplasia related to NRH should present an evident nodular staining pattern that matches reticulin staining (Figure 12).
Figure 12. Immunohistochemical staining for cytokeratin 7. The hypotrophic hepatocytes that border NRH nodules can present a biliary phenotype (cytokeratin 7 expression, so-called biliary metaplasia) in response to reduced blood flow.
Elastic-trichrome: stain to stage parenchyma fibrosis and rule out cirrhosis is required to diagnose PSVD. Elastic-trichrome combines a classic trichrome (Masson's or Mallory's) with Verhoeff's Van Gieson stain to highlight elastic fibers over the parenchymal fibrosis10. Remarkably, dark/black elastic fibers within the fibrotic tissue represent a chronic process11,12, thus allowing us to evaluate the overall parenchymal fibrosis and contemporarily date its temporal development (Figure 13).
Figure 13. Details of elastic-trichrome stain in two cases with increased portal fibrosis. Panel A shows the portal tract of a case with long-standing fibrosis; several black-stained elastic fibers are evident (black arrowheads) with a typical "undulated" shape. For comparison, panel B represents a case with increased but recent portal fibrosis with no elastic fibers.
***
Non-specific histology signs of PSVD
The following is a list of additional portal/periportal and lobular histopathological changes that can be observed on PSVD liver biopsy. However, according to the new diagnostic algorithm, they are not specific to PSVD, and their presence alone is insufficient for a definitive diagnosis of PSVD (see Diagnostic Algorithm paragraph).
1. Hypervascularized portal tract: single or multiple thin-walled vascular spaces in the connective tissue of the portal tract (figures 6 and 8).
2. Periportal abnormal vessels: single or multiple thin-walled vascular spaces outside the connective tissue of the portal tract that are confined to the periportal area (Figures 5-8).
3. Herniated portal vein branches: extensions of the portal vein outside the connective tissue of the portal tract toward the periportal hepatocytes (Figure 8).
4. Irregularly distributed portal tracts and central veins: the distance between portal tracts and central veins is usually within the range of 0.8-1.5 mm; changes in the intrahepatic vascular flow alter hepatocyte trophism and result in an irregular distribution of portal tracts and central veins throughout the liver parenchyma with or without parenchymal fibrosis (Figure 1).
***
Newly introduced diagnostic algorithm: modifications to the previous approach, and their relevance in the pediatric setting
Following the identification of histopathological changes and after excluding cirrhosis and other diseases specifically targeting liver microvascular structures, a diagnosis of PSVD can be made in the presence of:
1. A specific histological lesion of PSVD;
2. A specific sign of portal hypertension (i.e., varices, portal hypertensive bleeding, porto-systemic collaterals);
3. Non-specific histological lesion of PSVD combined with a non-specific sign of portal hypertension (i.e., ascites, platelet counts <150.000 per uL, splenomegaly).
A consideration needs to be highlighted: portal hypertension per se is not required for diagnosing PSVD. This criterion was implemented to support the identification of early-stage cases in which portal hypertension may not be fully developed/clinically evident, but histological lesions can already be visible on liver biopsy. A recent study on a pediatric population of 62 patients with PSVD and a 7-year follow-up period outlines the relevance of this aspect13. In this study, two sub-groups of patients were identified: the majority of cases (36 of 62, 58.1%) presented with portal hypertension and were associated with disease progression. However, the second subgroup did not present signs of portal hypertension or develop them during the follow-up period. Of note, specific and non-specific histologic changes were present in all patients, but portal vein stenosis was more frequent in the subgroup with portal hypertension, whereas patients with PSVD without portal hypertension presented more hypervascularized portal tracts.
***
PSVD: open questions and future directions
In conclusion, in this "pathology pearl" post, we revised the histopathology of PSVD according to the newly introduced definitions and diagnostic criteria. Following the recent revision, several questions need to be answered appropriately, ranging from a more granular analysis of the phenotype and temporal development of the disease to a more definite assessment of the pathophysiology and contribution of concurrent liver diseases. Future studies and a thorough comparison with the existing literature are required to complete our understanding of this multifaceted disease and improve patients' diagnostic and clinical management.
If you are eager to learn more about the potential clinical scenario, imaging features, and therapeutic implications of PSVD patients, please consider the recent post of the "Why? Series" published by Dr. Rabiee for non-cirrhotic portal hypertension14.
***
Tags: porto-sinusoidal vascular disease; portal vein stenosis; nodular regenerative hyperplasia; incomplete septal fibrosis.
Written by [Alessandro Gambella], [MD, PhD candidate], [Division of Liver and Transplant Pathology, University of Pittsburgh – Research Histology Services, Thomas E. Starzl Transplantation Institute]
***
References
1. Resnick, E.S., Moshier, E.L., Godbold, J.H. & Cunningham-Rundles, C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood119, 1650-1657 (2012).
2. Michel, M., et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood 114, 3167-3172 (2009).
3. De Gottardi, A., et al. Porto-sinusoidal vascular disease: proposal and description of a novel entity. Lancet Gastroenterol Hepatol 4, 399-411 (2019).
4. De Gottardi, A., Sempoux, C. & Berzigotti, A. Porto-sinusoidal vascular disorder. J Hepatol 77, 1124-1135 (2022).
5. Guido, M., et al. Histology of portal vascular changes associated with idiopathic non-cirrhotic portal hypertension: nomenclature and definition. Histopathology 74, 219-226 (2019).
6. Crawford, A.R., Lin, X.Z. & Crawford, J.M. The normal adult human liver biopsy: a quantitative reference standard. Hepatology 28, 323-331 (1998).
7. Reshamwala, P.A., Kleiner, D.E. & Heller, T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology 44, 7-14 (2006).
8. Sciot, R., et al. Incomplete septal cirrhosis: histopathological aspects. Histopathology 13, 593-603 (1988).
9. Delladetsima, I., Sakellariou, S., Kokkori, A. & Tiniakos, D. Atrophic hepatocytes express keratin 7 in ischemia-associated liver lesions. Histol Histopathol 31, 1089-1094 (2016).
10. Garvey, W. Modified elastic tissue-Masson trichrome stain. Stain Technol 59, 213-216 (1984).
11. Sato, S., et al. Abnormal elastic system fibers in fibrotic human liver. Med Electron Microsc 33, 135-142 (2000).
12. Hall, A., et al. Collagen and elastic fibres in acute and chronic liver injury. Sci Rep 11, 14569 (2021).
13. Di Giorgio, A., et al. Paediatric porto-sinusoidal vascular disease: Two different clinical phenotypes with subtle histological differences. Liver Int 43, 1523-1536 (2023).
14. Rabiee, A. Why is it important to think about non-cirrhotic portal hypertension? (2023).