PTLD: A Balancing Act of Immunosuppression
What is PTLD?
Post-transplant lymphoproliferative disorders are lymphomas in transplant recipients, first recognized in 1969 by Drs. Penn and Starzl. Fever (57%) and lymphadenopathy (38%) are common symptoms, and lab abnormalities can mimic rejection. Extra-nodal involvement, as was seen in this patient, is common, most frequently involving the GI tract (27%). The prevalence of PTLD is increasing, likely from increased numbers of transplants, heightened awareness of the disorder and improved diagnostics, newer potent immunosuppressive agents, and longer-term survival and follow-up.
What are risk factors for PTLD?
Type of transplanted organ
Rates vary based on the organ transplanted and is likely correlated with the amount of lymphoid tissue present as seen below in Figure 1. The highest risk is shortly after transplant, with one systematic review showing median duration from time of liver transplant to development of PTLD of 35 months in adults.
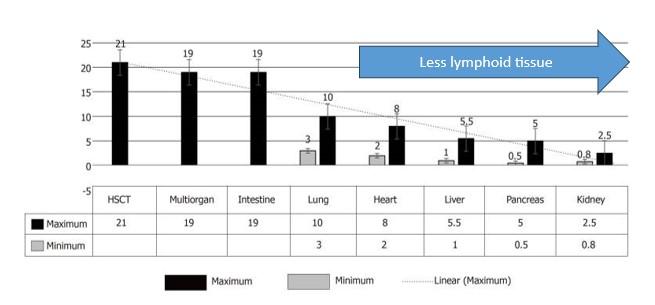
Figure 1. Incidence of PTLD in various transplants. Image from Abbas, et al. World J Transplant, 2020.
EBV infection
About 50% of cases of PTLD are attributed to EBV. The pathogenesis is likely due to EBV’s effects on both T and B lymphocytes in the setting of immunosuppression, immortalizing B cells and preventing T cells from killing infected cells. Mismatched EBV status (donor positive, recipient negative) and primary EBV infection in the host are highest risk, the latter of which can explain higher rates of PTLD in children, who are less likely to previously have been infected.
It is important to note that although PTLD can be the most devastating consequence of EBV, the virus can also cause numerous other syndromes, including the typical infectious mononucleosis or organ-specific inflammation (hepatitis, pneumonitis, or cytopenias).
EBV-negative PTLD occurs later after transplant (generally 5-15 years), likely due to long-term immune dysregulation leading to abnormal B cell proliferation. Infectious triggers including transient EBV infection or immune dysregulation from CMV have also been postulated although are not clearly linked. The prevalence of EBV-negative PTLD is increasing and nearing 50%, likely due to longer-term survival and follow-up among transplant recipients.
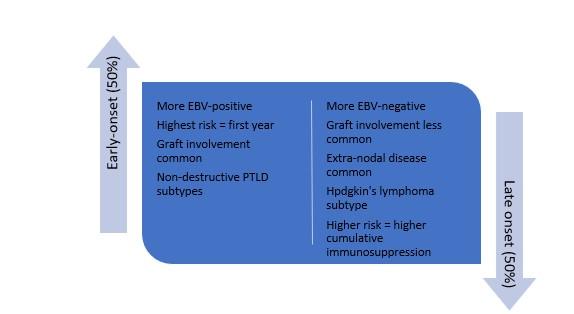
Table 1. Comparison of early versus late-onset PTLD. Adapted from Abbas et al, World J Transplant, 2020.
Immunosuppression
Another major risk factor for PTLD is the degree and length of immunosuppression. A clear association with certain immunosuppressing agents is difficult to study as patients are often on multiple agents prior to their diagnosis. Treatment for rejection was associated with higher rates of PTLD, especially in the first year after treatment. As above, CMV infection may play a role owing to its immunomodulating effects.
How is PTLD diagnosed?
Full-body CT and PET-CT scans are useful for staging and to help plan sites for biopsy. Histopathologic sampling is required for diagnosis. The World Health Organization created a classification system in 2017 as shown below in Figure 2.
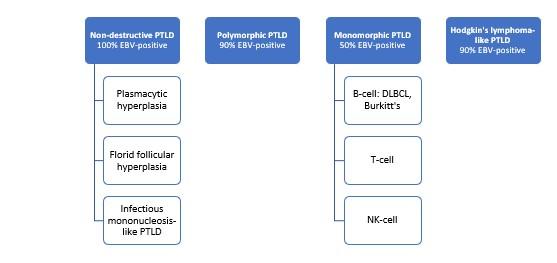
Figure 2. 2017 WHO PTLD Classification with associated rates of EBV positivity.
Determining EBV status by viral load and serology is recommended in all cases although is not required for the diagnosis and can be unreliable. False negatives can be seen in setting of immunosuppression, and false positives from passive transfer from blood products peri-transplant. Furthermore, viral load analyses are not uniform or standardized between institutions but could be monitored for trends over time.
Treatment
Reduction in immunosuppression leads to regression in many cases, with more success seen in EBV-positive cases. The rate of response is rapid, often seen in 2-4 weeks. It is recommended to cut the dose of calcineurin inhibitors (eg tacrolimus, cyclosporine) in half and discontinue anti-metabolites (eg azathioprine, mycophenolate mofetil). As expected, this can lead to rejection in over 1/3 of patients, thus graft function should be monitored closely.
Rituximab monotherapy, as well as in combination with CHOP therapy (cyclophosphamide, doxorubicin, vincristine, and prednisone) has been used, as well as surgery and radiation in certain circumstances. Chemotherapy can be considered in those with CNS disease, later-onset or EBV-negative disease, or in whom reduction in immunosuppression is not sufficient. Several future therapies are promising as seen in Table 2. Mortality rates are about the same between EBV-positive and negative cohorts, with a 50% survival at 5 years.
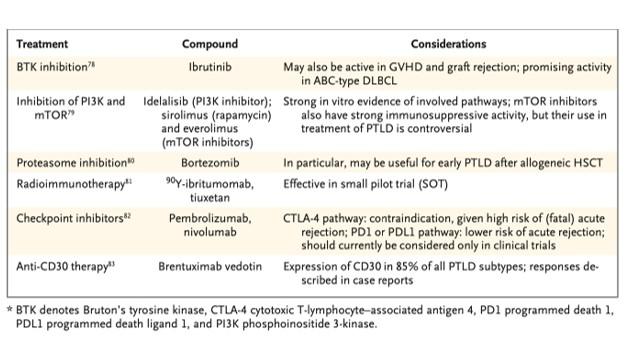
Table 2. Future strategies for treatment of PTLD. Image from Dierickx and Habermann, NEJM, 2018.
Prevention
A general guideline is to limit immunosuppression as able, and identify those at highest risk for development of PTLD, namely those with EBV serodiscordance (D+/R-) or with CMV infection/disease where more intensive monitoring may be warranted. There are no current consensus recommendations on antiviral prophylaxis with ganciclovir or acyclovir, or with IVIG administration.
Take Home Points:
- Post-transplant lymphoproliferative disorders are lymphomas related to immunosuppression, with the highest risk in the first several years after transplant.
- About half are associated with EBV, mostly occurring in the first year after transplant.
- Later-onset PTLD is more likely to be EBV-negative and is likely related to cumulative immunosuppression and ensuing immune dysregulation.
- The mainstay of treatment is reducing immunosuppression (generally cutting calcineurin inhibitors in half and discontinuing anti-metabolites), putting patients at high risk for rejection.
- Further treatment including chemotherapy is guided by specific PTLD subtype, with many promising therapies in development.
