Why do we use Lactulose and Rifaximin for Hepatic Encephalopathy?
Lactulose and Rifaximin (Xifaxan) are standards of care for the prevention of overt hepatic encephalopathy in patients with cirrhosis.
Have you ever wondered why? Join us!
First, if you want a refresher on hepatic encephalopathy (HE), check out our “Quick Tips” post by Lizzie Aby.
What is the first step in treating HE?
Reverse the underlying cause!!!
Infections, GI bleeding, medications, and dehydration can precipitate HE and treating these factors is most important in reversing an acute episode of HE.
So what next?
Medical therapy!

Lactulose, a non-absorbable sugar composed of galactose and glucose was first made in 1929. It was first used in 1966 by Bircher et al. to treat HE:

In this case report, the authors describe the proposed mechanism of action for the drug:
“The non-absorbed lactulose passes intact into the ascending colon, where it is split by bacterial action into lower molecular organic acids (mainly lactic acid and acetic acid). Their osmotic effect creates a “fermentive diarrhea” identical to the lactose-induced diarrhea in lactase-deficient subjects. The lowered fecal pH caused by the presence of these organic acids should hypothetically entail a change in colonic bacterial flora or a change in bacterial metabolism. An increase in fermentation and a decrease in putrefaction is to be expected; this should decrease the amount of nitrogenous compounds which are “toxic” in the portal-systemic encephalopathy.”
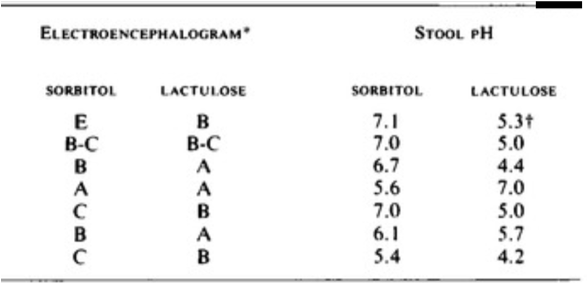
Another study published in 1969 by Elkington et al. further elucidated the mechanism of lactulose therapy. In this study, 10 patients with HE, all of whom had been treated by dietary protein restriction and prolonged administration of neomycin, were treated with lactulose or sorbitol (an osmotic laxative) and then were crossed over. The authors found that HE that was observed on sorbitol could be reversed by lactulose. Though both drugs are laxatives, the difference was that lactulose lowered stool pH!
As it turns out, not much has changed in terms of our understanding of the mechanism of the drug. It is thought to reduce the intestinal production/absorption of ammonia by several mechanisms:
- Colonic breakdown of lactulose to lactic acid leads to acidification of the gut which favors the formation of nonabsorbable NH4+ from NH3, thereby trapping NH4+ in the colon and reducing plasma ammonia concentrations.
- Colonic acidification displaces urease-producing bacteria with non-urease producing Lactobacillus.
- Lactulose works as a cathartic agent with a hyperosmolar load, leading to frequent bowel movements, with less time for ammonia absorption and increased fecal nitrogen excretion.
- Lactulose can have a prebiotic effect even at low doses by increasing the relative amount of bifidobacterial and lactobacilli and decreased branched-chain fatty acids.
- Lactulose can decrease bacterial-DNA translocation, perhaps by accelerating intestinal transit and improving bacterial overgrowth and intestinal permeability.
One of the first trials of lactulose was published in Gastroenterology in 1970 by Simmons et al. In this double-blind clinical trial, 11 patients with HE received 80g of lactulose daily while 10 patients received 60g of glucose daily as a control. Patients receiving lactulose demonstrated a significant reduction in HE and blood ammonia levels compared to controls. Interestingly, several patients dropped out of the study and the final analysis included only 4 patients in the lactulose group and 10 in the control group.
In a recent meta-analysis and systematic review, lactulose was found to prevent the risk of developing serious liver-related adverse events and reduces mortality.
Because of these studies and because of its success in clinical practice, lactulose is currently considered to be the “gold standard” for the treatment of acute episodes of hepatic encephalopathy. In the acute setting, lactulose is usually given until encephalopathy begins to improve. If the patient is able to swallow, it is administered orally, but it can also be administered via OG or NG tube, as well as rectally. Once acute encephalopathy is resolved, lactulose is given to target 2-3 bowel movements per day.
Lactulose is associated with several side effects, however, that make it intolerable to many patients. Because it is non-absorbable, it can be associated with flatulence, abdominal discomfort, bloating, and diarrhea. In patients with cirrhosis who are already quite sick and prone to electrolyte abnormalities, excessive diarrhea caused by lactulose can exacerbate hypokalemia and lead to hypernatremia. Additionally, because of the frequency of bowel movements that lactulose causes, some patients may experience perianal irritation. Because of these side effects, many patients are unable to be compliant with lactulose therapy. Patient compliance may also be impacted by socio-cultural factors as well as its perceived benefits.
So doc, what else can I use for an acute episode of HE?
Given that the pathophysiology of lactulose is at least partly based on the displacement of urease-producing bacteria, several oral antibiotics (neomycin, paromomycin, vancomycin, and metronidazole) have been studied based on their ability to similarly reduce ammonia-producing enteric bacteria. Most of these antibiotics when taken long-term, however, are associated with side effects, including nephrotoxicity, ototoxicity, and peripheral neuropathy. Additionally, taking systemic antibiotics long-term can be associated with antibiotic resistance, and remember, we are talking about a population that is already prone to infection and resistant organisms. Note: there are other therapies for HE that will not be discussed in detail for the purposes of this post. Briefly, outside of the US, oral L-ornithine-L-aspartate (LOLA) is sometimes given to patients for HE. Branched-chain amino acids, prebiotics, probiotics, and zinc have also been tried. All of these treatments require further study prior to their regular use to treat HE.
Now that I’ve been treated, how do I prevent another episode of HE?
While lactulose is the gold standard to treat overt HE, we know that for those on lactulose monotherapy, there is a 40% cumulative risk for recurrent overt HE within 6 months of an initial episode. AASLD and EASL both recommend secondary prophylaxis with rifaximin to reduce the recurrence of another HE episode while on lactulose.
Here comes RIFAXIMIN!!!!

Rifaximin is administered twice daily at a dose of 550mg. It is often started after the resolution of an acute episode of HE, sometimes even in the hospital. Its benefits compared to other antibiotics are that it is minimally absorbed, it is concentrated in the GI tract, and has a low risk of inducing bacterial resistance.
How does it work?
Surprisingly, the mechanisms behind rifaximin’s efficacy are poorly understood. It appears to be effective against a broad array of bacteria from gram-positive to gram-negative to aeorobic and even anaerobic organisms. Studies conducted by HE expert Jasmohan Bajaj (@JasmohanBajaj) have looked at microbiome changes associated with the use of rifaximin. One such study found that there was a shift from pathogenic to beneficial metabolite linkages and that this was associated with better cognition. Another study conducted in germ-free mice, however, demonstrated that rifaximin may impact ammonia generation and cecal amino-acid profiles even in the absence of microbiota changes. This indicates a potential direct effect of rifaximin on the host. Much more remains to be learned about the mechanism of action of this drug.
What’s the clinical evidence for rifaximin?
A landmark study in NEJM demonstrated that over a six-month period, treatment with rifaximin maintained remission from HE more effectively than placebo. Let’s examine this trial more closely:
Observational data also suggests a benefit of rifaximin. In a follow-up study to the original rifaximin trial, rifaximin continued to reduce the rate of HE-related and non-HE-related hospitalizations. Data published by Tapper et al. from United States Medicare enrollees, demonstrates rifaximin was associated with lower mortality, fewer thirty-day readmissions, and fewer hospital days in combination with lactulose. Similarly, a multicenter study conducted in the UK demonstrated that treatment with rifaximin was associated with a reduction in the number of hospital admissions and length of stay, thereby decreasing cost.
A meta-analysis of 19 studies comparing rifaximin versus placebo or other interventions (lactulose or other antibiotics) found that rifaximin had a benefit on recovery from HE, prevention of recurrence, and on mortality (see the risk ratio for mortality benefit below).
Though rifaximin’s only approved indication is for prevention of recurrence of HE, it has been used in other settings:
- Rifaximin has been studied for its use in acute overt HE, though it is not FDA approved for this purpose.
- Rifaximin can be beneficial in patients with minimal HE both in improving cognitive function as measured by neuropsychometric testing and in improving health related quality of life, though another study demonstrated that it was not effective in reversing minimal HE or improving quality of life.
So if rifaximin is so great, what is the elephant in the room??
There is really one simple answer, and that is COST!
The cost of a one-month’s supply of the drug could cost upwards of $1,000-$2,000 and for many, this is not covered by insurance. In comparison, the cost of lactulose is closer to $10-$20. The FDA actually just extended rifaximin’s patent protection until 2029. Despite the strong evidence that rifaximin works to prevent recurrence of HE, reduce risk of readmission, and improve mortality, the continued high cost of this drug will make it inaccessible to many. There have been multiple cost-effectiveness studies conducted demonstrating that with the current pricing, there are cost savings associated with the addition of rifaximin therapy owing to decreased HE-associated hospital admissions and hospital days.
What are potential side effects associated with rifaximin?
There are other theoretical risks associated with long-term rifaximin use including the possibility of bacterial resistance or C diff infection, but these events are actually quite rare. Plasma drug levels are really quite low even in patients with cirrhosis who have altered metabolism of drugs.
What else can we use rifaximin for?
Because of its favorable safety profile and because it is generally well tolerated in patients with cirrhosis, rifaximin is under investigation for additional therapeutic targets other than in HE. There has been some work looking at rifaximin’s potential role in reducing liver fibrosis, lowering portal hypertension, and preventing spontaneous bacterial peritonitis (SBP) or acute kidney injury, including hepatorenal syndrome. As was pointed out by an editorial written by Woodhouse and Shawcross, all of these ideas require rigorous further study given they are limited by biases intrinsic to their study designs.
All in all, the treatment of HE has come a long way in the past fifty years. There is much to be learned about the mechanistic effects of the medications we use and in making treatment affordable and accessible to all!




