Why machine perfusion has the potential to change the liver transplant landscape - and why hasn’t it yet?
Why has liver transplantation grown significantly in recent decades?
Orthotopic liver transplantation (OLT) has been performed since 1963 with more routine application being used starting since the 1980s. Over recent decades, OLT has dramatically grown as a cure for end-stage liver disease, increasing from 949 in 2005 to over 10,000 liver transplants in 2023. In light of this growth, the transplant process has adapted many changes affecting organ allocation and prioritization such as changes in the MELD scoring system, the introduction of living donor transplantation, and use of marginal or extended-criteria for transplant. Despite these efforts, or in light of them, there has been a growing waitlist for patients awaiting liver transplant mirrored by a population that is aging and becoming more obese, leading to a discrepancy between demand for organs and limited supply of standard-criteria organs. One area of increasing research in liver transplant that has the potential to increase transplants and decrease wait time and wait-list mortality is machine perfusion.
Why Are Static Cold Storage (SCS) and Its Limitations a Concern?
Static cold-storage (storing at low temperature between explanting and implanting) became standard for organ preservation for OLT in the early 1990s, and is sometimes referred to as “ice-box” storage. Static cold storage (SCS) involves flushing the donor liver with cold preservation solution via the hepatic artery and portal vein followed by placing the organ into a cold preservation solution and packing it in an ice box for transport. Cooling the organ allows for the reduction in metabolic demand. Graft function and survival post-transplant decrease after 8 to 12 hours.
While SCS provides for an environment with a reduction in hepatic metabolism, organ damage cannot be avoided and ischemia-reperfusion injury may occur. The goal with SCS is to reduce donor warm ischemia time to avoid ischemic injury. Enter machine perfusion.
Does Machine Perfusion Improve Outcomes?
Machine perfusion, or “liver pump,” refers to the technique of preserving organs during the transplantation process with circulating perfusate that mimics blood flow.
There are multiple ongoing randomized control trials looking into machine prefusion for liver transplantation though much of the current evidence is from cohort studies. Thus far, evidence from extant studies does suggest benefit over static cold storage, though there is still ongoing debate about machine perfusion strategy and overall outcomes.
A Cochrane review with seven randomized trials showed potential benefit as well with three trails looking into normothermic machine perfusion and four into hypothermic machine perfusion. There was no statistical improvement in mortality found and no quality of life data reported. However, with hypothermic machine perfusion there was improved graft survival and decreased adverse events in extended criteria grafts, as well as improvement in clinically significant ischemic cholangiopathy in recipients of DCD livers, compared to SCS. Normothermic machine perfusion was associated with improved utilization in grafts that would be otherwise discarded, though this effect warrants further investigation.
This study of 50 patients showed improvement in graft survival with hypothermic machine perfusion including 5 year graft survival. A randomized control trial of normothermic machine perfusion with 220 patients showed improvement of graft injury by 50%, even with a 50% lower rate of organ discard, though failed to show improvement in graft or patient survival or cholangiopathy.
Thus while evidence is optimistic, more data and specifically randomized control trials are needed to help guide the field to confirm safety, efficacy, optimization, and cost-benefit analysis.
Why is machine perfusion gaining attention in liver transplantation?
This remains a growing field or research with many logistical and economic questions left to answer including device and process optimization and viability testing as well as policy changes. In 2021, two devices were FDA approved as normothermic machine perfusion devices for the purpose of continuing perfusion on explanted livers prior to transplantation. The use of these devices has broadened the ability to make more organs available for transplantation, though the use of machine perfusion is not yet a ubiquitous standard of care.
This is not a strictly novel approach to transplant, however. Machine perfusion was first researched 50 years ago for kidney transplant with the first successful perfusion pump by Nobel Prize–awarded French physician Alexis Carrel and the American pilot‐engineer Charles Lindbergh.
The methodology of machine perfusion can be broken down into two parts: (1) temperature and (2) timing of the perfusion. The subsequent sections detail the various methods of machine perfusion including normothermic and hypothermic machine perfusion and their timings.
Why does temperature matter?
The temperature of the perfusate is tied to liver metabolic function. In general, this can be broken down into hypothermic and normothermic, with hypothermic being less than 12°C and normothermic being 35°C to 37°C. Hypothermic perfusion can result in a 6–12‐fold metabolic suppression, whereas normothermic perfusion necessitates high metabolic support but allows for assessment of viability.
Why Does Normothermic Machine Perfusion (NMP) Improve Liver Transplant Outcomes?
Normothermic machine perfusion (NMP) for OLT was first successfully performed in 2012. Because normothermic perfusion (ex situ) requires higher metabolic support through nutrients and oxygenation, perfusion machines need dual perfusion lines for the portal vein and hepatic artery with oxygenation sensors. In contrast to SCS, NMP reduces injury and ischemia duration. Advantages of NMP include potentially lower levels of bile and factor V production, glucose metabolism and galactose clearance, lower levels of hepatocellular enzymes in perfusate compared to cold stored livers, and the ability to assess graft viability before transplant. Some research is ongoing looking into avoidance of any cold storage or perfusion through normothermic perfusion techniques or an “ischemia free organ transplantation.”
Why is Hypothermic Oxygenated Perfusion (HOPE) a Promising Alternative to Cold Storage?
In contrast, hypothermic machine perfusion (HMP) can be performed with lower required levels of oxygen. Hypothermic Oxygenated Perfusion (HOPE) requires short metabolic conversion time and is usually sufficient for 1-2 hours before implantation for end-ischemic perfusion strategy though can be performed the entire time between explant and implant. This can be accomplished successfully via portal vein alone, though can also be accomplished with dual-HOPE using both portal vein and hepatic artery. There is some evidence that HOPE helps to lower the risk of ischemia reperfusion injury (IRI) and reduces post-transplant complications such as cholangiopathy through protection of the mitochondria. IRI is the result of transitioning from anaerobic metabolism with energy loss and accumulation of toxic metabolites to an aerobic environment with normothermic conditions leading to release of mitochondrial reactive oxygen species. Hypothermic reoxygenation protects against this. Hypothermic machine perfusion protection against IRI is likely multifactorial involving slowing cellular metabolism, removal of metabolic products via perfusion, and delivery of oxygen.
Why is Normothermic Regional Perfusion (NRP) Used in Donation After Circulatory Death (DCD)?
There is also normothermic regional perfusion (NRP) which is different from NMP as it is in situ. NRP occurs immediately after warm ischemia in DCD donors. The benefit of this strategy is elimination of cold ischemia at time of normothermic reperfusion, in addition to being less costly and with potential benefit to other organ grafts. Downside to this strategy include longer operating room time and affect on other abdominal organs. This is also the most costly and labor intensive of the perfusion techniques.
Here is a graphic representation of these various methods from Ceresa et al.
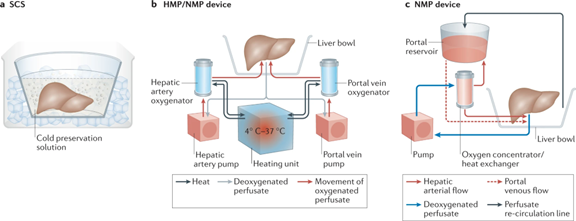
Below is an abbreviated table from Schlegel et al summarizing various perfusion techniques.
| Technique | Benefits | Mechanisms | Opportunities | Challenges |
| Normothermic regional perfusion (NRP): immediate recirculation of donor blood in situ as initial step of organ procurement, for 2-4 h at 37 °C, followed by cold in situ flush, liver removal and transport | Early reintroduction of oxygen | Immediate IRI and release of proinflammatory and organ-specific molecules, e.g., DAMPs, cytokines, lactate, liver enzymes | Assess viability, increase utilization, combination with ex situ machine perfusion for additional treatment and viability testing | Only for specific donor types (e.g., DCD) |
| Early liver evaluation | Logistically difficult to prolong to apply specific treatments | |||
| Perfusion of more than one organ Physiologic liver position and communication with other organs | Consensus needed for different abdominal organs | |||
| No viability testing after additional cold storage | ||||
| Normothermic machine perfusion (NMP): ex situ liver perfusion at 37° with a blood-based perfusate (or oxygen carrier), applied after cold liver flush and procurement, during or after transport to recipient center for 4-24 h | Reduces injury when applied instead of cold storage | IRI and release of proinflammatory and organ-specific molecules, e.g., DAMPs, cytokines, lactate, transaminases | Assess viability (e.g., perfusate, bile) | Device transport |
| Reduces ischemia (= hypoxia) duration with reduced liver injury at transplantation (e.g., EAD) | Increase utilization | Costs and labour intensity | ||
| Prolong preservation for optimized logistics | Validate viability markers less effective with previous cold storage before NMP | |||
| Hypothermic oxygenated perfusion (HOPE, D-HOPE, HMP-O2): ex situ liver perfusion at 8-12 °C with a highly oxygenated (PO2>80 kPa) artificial perfusion solution, after standard procurement, during or after transport for 2–24 h | Prepares livers (mitochondria) for reperfusion at normothermic conditions Reduces IRI, improves post-transplant outcomes (liver function, graft survival, reduced complications) Applied after cold storage | Reprogramed mitochondria (re-activate respiration without significant ROS release, reduce toxic metabolites, recharge ATP) | Assess viability (perfusate) | Additional costs compared to cold storage |
| Increase utilization | Validate viability markers | |||
| Prolong preservation for optimized logistics | ||||
| Combination with NMP to reduce IRI-inflammation |
Why does timing matter?
Machine perfusion can be performed at three time points; (1) immediately afterorgan procurement, before static cold storage, (2) shortly prior to organ implantation, and (3) for the entire preservation period between procurement and implantation. Schlegal et al describe below in the following diagram the complexity of the potential perfusion timing logistics.
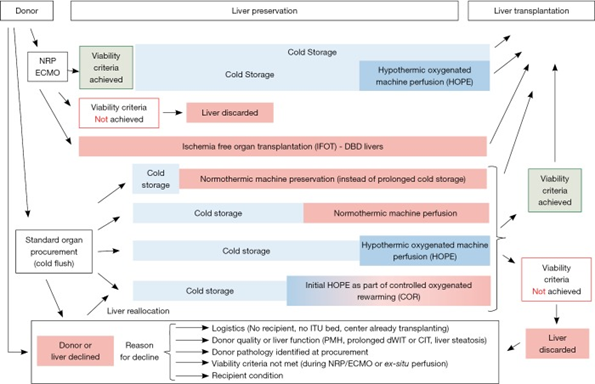
For normothermic perfusion, the device can be applied during or immediately after procurement at the donor site. Normothermic perfusion typically ranges between 3 and 16 hours, though there are some instances of long-term perfusion which have lasted as long as a week. There is ongoing research into if prolonged machine perfusion can limit ischemic reperfusion injury, though barriers still include technical demands and procedures and cost associated. For donor sites without NMP capability, an alternative is standard static cold storage followed by normothermic perfusion, which is known as “end-ischemic” perfusion. Hypothermic machine perfusion is applied prior to implantation.
Why is Assessing Liver Graft Viability Crucial Before Transplantation?
While donation after brain death (DBD) organs are lower risk compared to donation after circulatory death (DCD) donors or donors with high degree of steatosis, assessment of viability at the end of cold static preservation can help to ensure safe transplantation. Utilizing the cold perfusion system and to measure viability markers could indicate which grafts could benefit from normothermic perfusion prior to transplantation and potentially increase utilization rates of higher risk grafts.
One of the potential benefits of machine perfusion is the ability to utilize extended criteria organs. Extended criteria can be broken down into graft dysfunction (older grafts, DCD grafts, or grafts with a large degree of steatosis or prolonged cold ischemia time) and disease transmission (positive hepatitis B core antibody, positive hepatitis C antibody, HIV status, and known history of malignancy).
Liver pumps are expanding the donor pool with extended criteria organs, in part, because of the ability to test for viability during NMP. This is beneficial as extended criteria organs are at higher risk of early allograft dysfunction, primary nonfunction, and biliary complications.While there is no reliable marker of liver graft viability, machine perfusion allows for an assessment to take place assessing organ appearance, consistency, hemodynamics, and metabolic and excretory function. Historically, graft viability has been assessed histologically and through analysis of serum markers such as transaminases and LDH.
NMP allows for viability assessment as the organ is maintained in a near-physiological state of oxygenated perfusion around body temperature. Viability criteria incorporate both hepatocellular and cholangiocellular criteria, in addition to liver function (lactate and ammonia clearance, pH maintenance, urea production), excretory function (bile production, drug clearance, coagulation factor production), graft appearance, hemodynamics (flow, pressure, resistance), and function of biliary epithelium (biliary levels of glucose, pH, bicarbonate). Lactate and bile production levels have been used to assess viability, though there is conflicting evidence as to whether this reflects liver organ function or confounders such as the ability to respond to vasoactive drugs or endocrine hormones. There is no currently agreed upon viability marker composition. The below chart from Brüggenwirth et al demonstrates the various viability markers during machine perfusion.
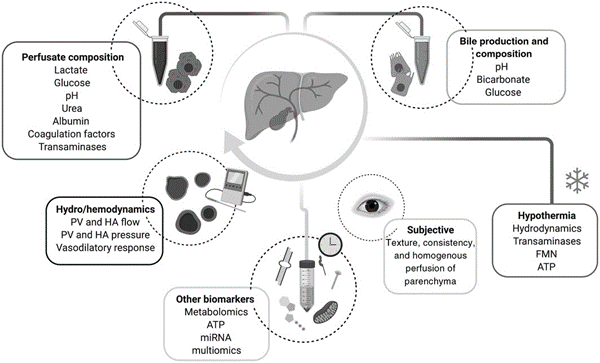
Viability is more difficult to measure during HMP given lower metabolic demand, though markers including hydrodynamics (flow and resistance), liver transaminases, and flavin mononucleotide (surrogate for mitochondrial injury) can be used.
Why does machine perfusion aid in organ demand?
One of the major benefits to the use of machine perfusion is the increase in graft viability. Over the last decade, transplant has been affected by two major problems: increase in high-risk donor grafts (aging population, increasing obesity) and increase in organ demand.
The percentage of discarded donor livers in the United States is expected to increase from 22% to 56% from 2010 to 2030 if practices were to remain constant without utilization of ex vivo perfusion techniques to increase the use of marginal donor livers. Moreover, there has been an increase in discarded grafts given low quality attributable to an aging and obese population. Similarly, much of the transplanted organs over recent years has been in higher risk donors including older donors with cardiovascular disease, diabetes, hepatic steatosis, and DCD donors. Marginal grafts, such as those with more significant steatosis or from donors with circulatory death, carry an increased risk of graft-associated complications, such as primary nonfunction, delayed graft function, or late biliary injuries. There are various risk scoring systems (UK DCD, Balance of Risk score system, Donor Liver Index, DCD-Risk Index) to weigh donor graft and recipient transplant risk, though they are limited by relatively low positive predictive value and given limited uptake of machine perfusion do not stratify for perfusion strategy.
Why Is Further Research Needed to Optimize Machine Perfusion Techniques?
There remains a dearth of research surrounding machine perfusion. Current research is promising, but questions remain. Current recommendations are based on cohort studies, though there are ongoing randomized control trials.
Machine liver perfusion is an area of growing research that has the potential to expand the marginal donor pool. Higher risk grafts are more susceptible to ischemia and reperfusion injury with SCS which remains an effective strategy for low risk grafts. As such, current practice with NMP mainly focuses on assessing organ viability and assessment of higher risk organs, in addition to aiding logistical time constraints for transplant. Machine perfusion helps to protect organs for reperfusion in addition to allowing for a viability assessment of a functioning ex situ organ. This allows for risk mitigation when transplanting an extended criteria liver. Furthermore, future efforts will allow for research into extended preservation times for more time to utilize therapeutic interventions such as fat removal or use of immunomodulators. The main question yet to be answered is which perfusion technique allows for the highest utilization rate of grafts with optimal outcomes that would have previously been discarded.
Given regional variability in donor profiles and center experience with machine perfusion, there is not yet standardize guidance on technique. There also is ongoing need for cost-benefit analysis as techniques become more refined and utilized.
While extant literature demonstrates how HMP improves transplant outcomes compared to SCS and NMP has potential to increase graft utilization, further studies are needed to evaluate comparison between perfusion technologies.
Take home points
- Machine Perfusion Enhances Organ Availability: Machine perfusion, particularly normothermic machine perfusion (NMP) and hypothermic oxygenated perfusion (HOPE), has the potential to help expand the donor pool by improving the preservation and viability of donor livers, especially marginal grafts.
- Cold Storage Limitations: Static cold storage (SCS), while effective for many livers, is limited by ischemia-reperfusion injury (IRI) and decreased graft survival after prolonged cold storage (8-12 hours), particularly for high-risk grafts.
- Normothermic Machine Perfusion (NMP): NMP, introduced in 2012, allows preservation of the liver at 37°C with oxygenated blood, providing the opportunity to assess organ viability and reduce ischemic injury, but is more technically demanding and costly.
- Hypothermic Oxygenated Perfusion (HOPE): HOPE uses cold (8-12°C) oxygenated perfusate, protecting mitochondria and reducing ischemia related injury, and is typically applied for 1-2 hours before implantation, offering a promising strategy for end-ischemic liver preservation.
- Normothermic Regional Perfusion (NRP): NRP, performed in situ on donation after circulatory death (DCD) donors, helps prevent cold ischemia injury and may reduce costs but is more labor-intensive and affects other abdominal organs.
- Viability Assessment with Machine Perfusion: Machine perfusion techniques enable real-time viability assessment of donor livers, including metrics like lactate and bile production, liver function, and hemodynamics, which help decide if a graft is suitable for transplantation.
- Increased Use of Extended Criteria Organs: Machine perfusion supports the use of extended criteria organs (e.g., older livers, DCD livers, or those with steatosis), reducing the risk of primary nonfunction, delayed graft function, and biliary complications.
- Ongoing Research Needs: While machine perfusion shows promise, further randomized controlled trials and studies are needed to refine viability markers, compare different perfusion techniques, perform cost-benefit analysis, and optimize graft utilization strategies.
- Barriers to Widespread Adoption: Despite its potential, machine perfusion is not yet a ubiquitous standard due to high costs, technical complexity, lack of consensus on best practices, and variability in regional donor and transplant center capabilities.
