Pediatric Hepatitis B
Background
Despite availability of an effective vaccine, hepatitis B virus (HBV) infection remains a worldwide public health issue with approximately 1.5 million new infections per year and an estimated 292 million chronic carriers. Although the virus can infect people of any age, those who are infected perinatally or as children are at greatest risk for chronic infection. Approximately 90% of those with chronic infection were infected perinatally, primarily in Asia (75% of chronic cases).Those with chronic infection are at highest risk of developing complications of the infection, including liver cirrhosis and hepatocellular carcinoma (HCC). Hepatitis B is the 2nd most common carcinogen worldwide after tobacco. Approximately 1 million people per year die from hepatitis B-related complications worldwide.
Rates of HBV infection vary substantially by geographic region. Worldwide, HBV infection prevalence is highest in the Asia, Africa, Southern Europe, and Latin America, where hepatitis B surface antigen (HBsAg) positive rates in the general population range from 2 to 20%. In countries where HBV is endemic, HBV infection occurs mainly during infancy and early childhood with perinatal transmission accounting for up to 90% of all chronic HBV infections. Perinatal transmission in non-endemic countries, including the United States, occurs most commonly in children of HBV-infected mothers who do not receive appropriate HBV immunoprophylaxis at birth.
Distribution of HBV infections worldwide

Image taken from: CDC.gov
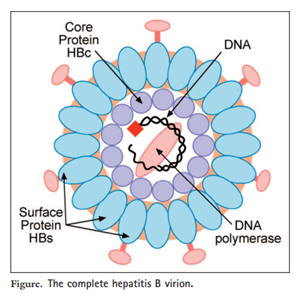
The hepatitis B viral particle is composed of an outer lipoprotein envelope, which contains hepatitis B surface antigen (HBsAg), and an inner nucleocapsid, which consists of hepatitis B core antigen (HBcAg).
Diagram of Hepatitis B Virus
Clinical Features

The incidence of acute HBV infection among US children and adolescents declined dramatically during the era of universal childhood vaccination, from 3.03 reported pediatric cases per 100,000 population in 1990 to 0.34 per 100,000 in 2002.Since then, surveillance data from the Center for Disease Control (CDC) from 2011 to 2020 has continued to demonstrate low acute HBV infection rates (0.0 cases per 100,000) among children age 0-19 years.
HBV infection in children has a variable course ranging from asymptomatic infection to fulminant hepatitis, with an incubation period of one to four months. Constitutional symptoms, anorexia, nausea, jaundice, and right upper quadrant discomfort can occur during the prodromal phase, and typically disappear after 1-3 months. Serologic testing is characterized by positive hepatitis B surface antigen (HBsAg) and IgM antibody to hepatitis B core antigen (IgM anti-HBc).
The proportion of patients progressing to chronic infection is much higher in neonates (up to 90 percent) compared with 1 to 5 percent in adults and intermediate values in young children. Chronic HBV infection is characterized by the persistent presence of HBsAg for at least 6 months. While HBV is not directly cytopathic, the host-mediated response to infected hepatocytes is thought to mediate chronic liver injury. In contrast with acute HBV, in chronic HBV infection total anti-HBc is usually positive (because of the immunoglobulin G [IgG] component), while IgM anti-HBc is negative.
Chronic hepatitis B infection is a dynamic disease traditionally divided into four phases, defined by the associated laboratory findings (as seen in the AASLD 2018 Guidelines).Of note, the European Association for the Study of the Liver (EASL)’s 2017 Clinical Practice Guidelines utilize a new nomenclature dividing natural history of chronic HBV infection into five phases based on HBeAg status, HBV DNA levels, ALT values, and the presence of absence of liver inflammation.
Indications for treatment
The goal of antiviral therapy for chronic HBV infection is to shorten the duration of liver inflammation and reduce the risk of progression to cirrhosis. Selection of patients for treatment is driven by the phase of HBV infection.
- Immune-tolerant phase: Anti-viral treatment during this phase is not recommended, as it does not result in high rates of HBeAg seroconversion compared with no treatment and is associated with development of drug resistance which could diminish responsiveness to therapy later.
- Immune-active phase: Anti-viral treatment is recommended for patients with persistently abnormal ALT (>2 times ULN) and HBV DNA >20,000 international units/mL for at least 4 to 6 months. If there is evidence of hepatic decompensation, such as jaundice or coagulopathy, treatment should be initiated earlier.
- Inactive hepatitis B phase: Anti-viral treatment during this phase is not recommended but patients should be continually monitored for reactivation.
- Treatment should be considered in those patients who are undergoing immunosuppression or cytotoxic therapy such as chemotherapy or organ transplants despite the phase of HBV infection.
Approach to treatment initiation of Hepatitis B in children.
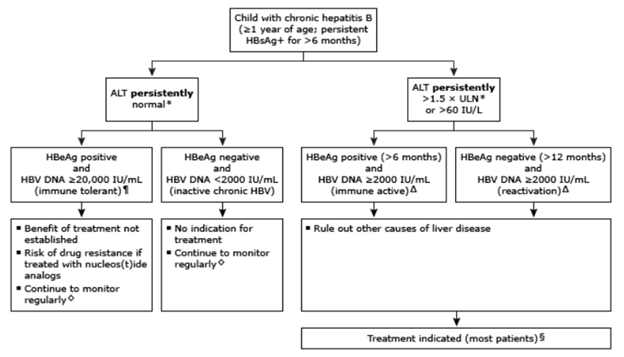
¶: Patients with normal ALT and intermediate HBV DNA concentrations (HBV DNA 2000-20,000 IU/mL) cannot be immediately categorized; suggest retesting in a few months. Δ: For the rare patient who has persistently elevated ALT but has low viral load (HBV DNA <2000 IU/mL), an evaluation for additional causes of liver disease is appropriate. ◊: For patients in the immune tolerant and inactive chronic HBV phases, we suggest monitoring liver biochemical tests (ALT and AST) every 6 to 12 months, and HBeAg and HBeAb every 12 months. If the ALT becomes elevated, HBV DNA should be measured. §: Treatment is indicated for most patients in the immune active, HBeAg positive phase. However, if the liver biopsy shows little or no inflammation or fibrosis, it is reasonable to defer treatment with ongoing monitoring.
Antiviral Therapy
Current treatment options for chronic HBV infection in children include interferon (IFN) or nucleoside analogs (entecavir and tenofovir). Any of the three medications can be used as first-line therapy, with selection based on age and preferences of the patient and family. Other drugs licensed for use in children such as lamivudine and adefovir are now rarely used due to limited efficacy and high rates of drug resistance.
Antiviral Medications for Pediatric HBV
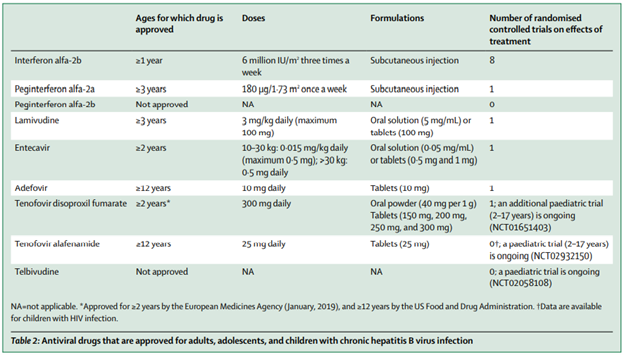
IFN has a finite duration of therapy (one year) but requires subcutaneous injections and is not recommended for patients with concomitant autoimmune disorder, organ transplant, decompensated liver disease, or serious neuropsychiatric disease due to its side effect profile. Potential treatment related adverse effects include flu-like symptoms (fever, myalgias, headache, anorexia), bone marrow suppression (typically neutropenia), behavioral symptoms (increased irritability), and weight loss.
Nucleoside analogs such as entecavir and tenofovir are good options for patients who prefer oral therapy. Patients may require longer-term therapy compared to interferon, as treatment duration depends on patient’s response to therapy. Entecavir has overall low rates of drug resistance in nucleoside-naïve patients but notes higher rates in patients previously exposed to lamivudine; tenofovir does not appear to induce viral resistance and is often effective in patients who developed resistance with other nucleoside analogs.
Prevention
The timing of the first dose of HepB vaccine and the need for hepatitis B immunoglobulin (HBIG) is determined by the mother’s hepatitis B surface antigen status and the infant’s birth weight.
For infants with HBsAg-positive mother or with other evidence of HBV infection (presence of hepatitis B DNA, positive HBeAg, known chronic hepatitis B infection), the first dose of single-antigen HepB vaccine and HBIG should be administered as soon as possible and within 12 hours after birth. This should occur regardless of birth weight. Infants who receive appropriate immunoprophylaxis may breastfeed immediately after birth.
For infants with birth weight >2 kg, two subsequent doses of the Hep B vaccine should be given at one month and six months of age, respectively. For infants with birth weight <2 kg, three additional doses should be given at one month, two to three months, and six months of age. Infants should undergo post-vaccination testing for HBsAg and antibody to HBsAg at 9 to 15 months of age.
For infants with HBsAg-negative mother and birth weight <2 kg, the infant should receive the first dose of HepB vaccine at one month age or at hospital discharge if discharge occurs before one month of age. For infants with HBsAg-negative mother and birth weight >2 kg, the recommended vaccine schedule is as follows:
First dose – During the birth hospitalization, within 24 hours of birth
Second dose – At 1 to 2 months of age
Third dose – At 6 to 18 months of age (must be given at ≥24 weeks of age)
