Pediatric Acute Liver Failure: from Prompt Recognition and Management to Liver Transplant Considerations
Defining Pediatric Acute Liver Failure
Pediatric acute liver failure (PALF) represents a medical emergency, characterized by the acute onset of hepatocellular damage manifesting as elevated hepatic transaminases and coagulopathies in a child without pre-existing liver disease. The PALF criteria was established by the pediatric ALF study group (PALFSG). Unlike the adult criteria, the diagnosis of PALF does not require the presence of hepatic encephalopathy (HE).
All three components are required in the diagnosis of PALF per the North American pediatric ALF study group:
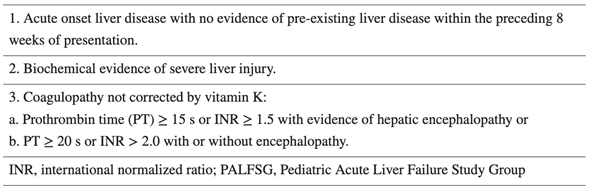
This algorithm describes the next steps of management of PALF:
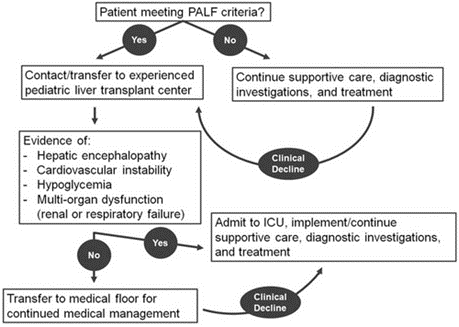
Urgency of Diagnosis
PALF is a rapidly progressive disease state that is associated with high mortality without urgent evaluation, management, and potentially liver transplantation (LT). A patient may quickly deteriorate within hours to days of presentation. Whether a child may spontaneously recover, require LT for survival, or die from complications of disease, the potential outcomes of PALF quickly become evidenced within the first week of hospitalization. Thus, prompt intervention and management may influence outcomes.
The fragile clinical state and associated high mortality of PALF within the first week are the basis for listing a patient as status 1A for liver transplant. The patient in the question stem meets criteria for PALF, given his elevated liver enzymes with lack of pre-existing liver disease, and INR value greater than 2.0.
LT, while potentially restorative to an otherwise decompensating patient, may also interrupt the natural course of a child in liver failure who may have otherwise survived without one. The clinical prodrome (typically of nonspecific symptoms like the patient in the question who presented with fevers, emesis, and fatigue) is followed by the potential for recovery or death, as depicted in the figure below.
Etiologies of PALF
The differential diagnoses for PALF are broad, ranging from infection, toxic exposure, autoimmune liver disease, and metabolic etiologies as shown below. A broad workup must be pursued to evaluate for underlying causes. In children, the leading cause of PALF is indeterminate, unlike the adult population where acetaminophen toxicity is the primary cause. Indeterminate PALF / activated T cell hepatitis will be discussed in a separate LFN post.
Outcomes of PALF vary by age and etiology. In the patient from the question stem, the most common etiologies in the differential would include indeterminate, acetaminophen toxicity, autoimmune hepatitis, and viral hepatitis.
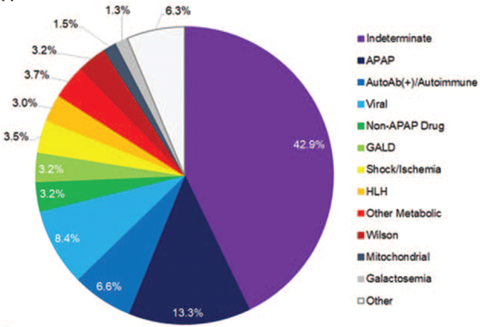
The cause of PALF is never found in 30-50% of cases.
Hepatic Encephalopathy
HE is challenging to diagnose in children – in this case, the patient’s confusion should prompt further evaluation for this condition. Neurocritical care can help delineate the presence and stages of HE with the assessment and use of transcranial doppler and/or video EEG.
Various scoring criteria are utilized to diagnose HE among children. For the patient in the question stem, his confusion and lack of orientation correspond with advanced HE (stage 3-4). In younger children, EEG testing may be a valuable tool for the patient to help determine the presence and stage of HE.
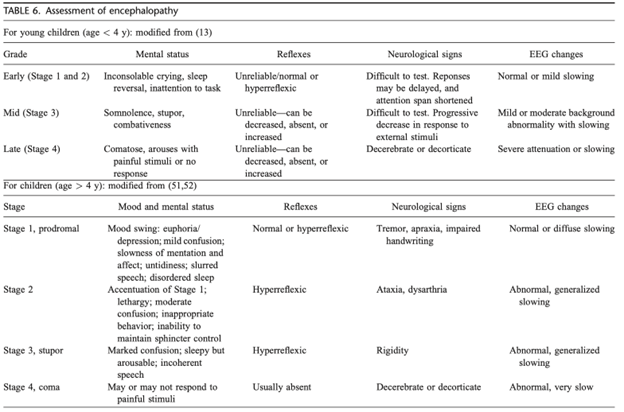
All PALF patients require close evaluation for presence and severity of HE, which may be challenging to assess in younger children. Mental status changes in the setting of electrolyte derangements and/or hemodynamic instability may influence the evaluation for HE. Thorough assessment is necessary as the presence and extent of HE can help dictate management and potential interventions. A child in liver failure requires serial examinations even if presenting initially with a normal mental status. Mental status changes, such as confusion, combativeness, excessive fatigue, and neurological changes, including brisk reflects and the Babinski reflex, require prompt recognition. Particularly in the case above, in which a child initially presents with HE, even more urgency is warranted. A study by the PALFSG demonstrated that mortality at 21-days was highest in patients with stage three or four HE or in those with progression of HE.
Ammonia plays a key role in monitoring and management of HE in PALF. Multiple toxins including cytokines, bile acids, chemokines, and ammonia likely contribute to HE. It is thought that the diffusion of ammonia across the blood-brain barrier interferes with the glutamine-glutamate cycle with subsequent osmotic edema from astrocyte glutamine. Arterial ammonia levels are most accurate, though venous ammonia (taken from a free-flowing catheter and immediately placed on ice) is an acceptable and often more feasible option. While a specific level of ammonia does not necessarily correspond with the degree of HE, an ammonia level greater than 100 μmol/L on admission is a risk factor for the development of high-grade HE, while a level greater than 200 μmol/L is associated with cerebral herniation. With rising ammonia levels, certain medical therapies become important (see below, medical management part 2). While Lactulose and Rifaximin are agents used in chronic liver disease or acute on chronic liver failure to treat hyperammonemia, there is low evidence to support their use in PALF.
See Liver Fellow Network post, “Quick Tips: Hepatic Encephalopathy” and “Just Another Case of Hepatic Encephalopathy,” for great overviews of HE presentation and management in adults.
Medical Management (Part 1)
Medical management typically includes immediate transfer to a liver transplant center pediatric intensive care unit (PICU), GI bleeding prophylaxis with a histamine blocker or proton pump inhibitor, continuous glucose infusion, fluid restriction, and avoidance of sedation. Specifically, benzodiazepines are not metabolized by a failing liver and may interfere with the crucial neurologic exam. As patients in liver failure are predisposed to bacterial infections in the setting of dysfunction of the immune system, antibiotics should be considered and administered with clinical signs and symptoms of sepsis. Management of PALF is largely supportive unless a specific etiology is uncovered that is amenable to targeted therapy, like autoimmune hepatitis, Wilson’s disease,herpes virus infection,tyrosinemia, or acetaminophen toxicity.
N-acetylcysteine (NAC), an antioxidant that renews glutathione stores, is used to treat acute acetaminophen toxicity. NAC has also been shown to improve transplantation-free survival among adults with ALF from non-acetaminophen causes and in those with grade 1-2 HE. Thus, a study was conducted to explore whether NAC would offer the same therapeutic benefit in children. The trial enrolled 184 children between the ages of birth through 17-years, allocating each patient into the NAC (150 mg/kg/day in 5% dextrose in water) versus placebo (5% dextrose in water) group for up to seven days. The study ultimately found that one-year survival did not significantly differ between the treatment versus placebo groups. Furthermore, in the evaluation of secondary outcomes, investigators found that one-year LT-free survival was actually lower in children who were given NAC compared to placebo, particularly among those younger than two years with HE grades 0-1: LT-free survival was 29% in the NAC group compared to 58% in the placebo group. As use of NAC did not improve 1-year survival, and actually lead to lower LT-free survival among children with non-acetaminophen causes of liver failure, this specific therapy is not supported for children with ALF, though it may be considered in adolescents who present with ALF from an unknown etiology with possible exposure to drugs and/or toxic ingestion. More to come on this in the next LFN PALF post.
“Correcting the Coagulopathy”
Among its many roles, the liver synthesizes most of the coagulation factors and helps maintain homeostasis. Given that there is a reduction in both anti and pro-coagulant factors in acute liver failure (see table below), a “rebalanced coagulation state” is thought to occur.
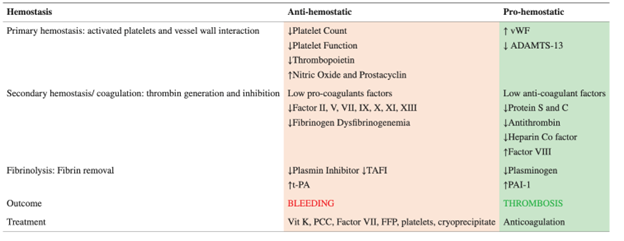
Bleeding is not common in PALF. Furthermore, the risk of bleeding does not necessarily correlate with international normalized ratio (INR). Thus, administration of products like fresh frozen plasma (FFP) should not be given to “correct the coagulopathy,” as it has not been shown to reduce the risk of bleeding and increases the potential risks of fluid overload, transfusion-related acute lung injury, hemolytic transfusion reactions, and elevated intracranial pressure and cerebral edema.
Cryoprecipitate is a plasma-derived product that contains fibrinogen, factors 8 and 13, vWB factor and fibronectin, and may be considered in PALF in the setting of low fibrinogen levels to reduce the risk of bleeding. Small volumes of cryoprecipitate are required to increase the fibrinogen level, lowering the risk of potential side effects.Product administration before a procedure or for clinically significant bleeding would be indicated.
Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are whole blood tests that measure the formation/degradation of a clot and may help determine risk of bleeding and guide administration of specific blood products accordingly. Guidelines with TEG and ROTEM in the management of PALF have not yet been established.
See the Liver Fellow Network posts, “Why (and how) does coagulopathy occur with liver disease” and “The Coagulopathy of Liver Disease – Part 2” for a fantastic and detailed overviews of the topic.
Medical Management (part 2)
Extracorporeal liver support systems (ECLS), like plasmapheresis/plasma exchange and molecular adsorbent recirculating system (MARS) have a role in PALF, though there is sparse literature on the associated outcomes. Thus, they are not routinely utilized.
Plasmapheresis may be utilized as a bridge to LT, and possibly to recovery in PALF. Given that the patient continues to deteriorate while awaiting transplantation, now with evidence of multi-organ failure, therapeutic plasmapheresis would allow for the removal of toxins in the blood and potentially create an environment in which the liver may be able to recover or alternatively stabilize the patient until they can receive a liver transplant. Additionally, plasmapheresis has the added immunomodulatory affect of removing proinflammatory cytokines. A randomized control trial among adults with ALF showed that a three-day course of plasma exchange improved transplant-free survival. Some pediatric studies have shown improvement in coagulation profiles with use of plasmapheresis, but not necessarily in transplant-free recovery.
MARS similarly functions to detoxify the blood but through a membrane with albumin-related binding sites that separate the patient’s blood from albumin dialysate, allowing for removal of albumin-bound products like bilirubin and aromatic amino acids as well as unbound low-molecular weight molecules like ammonia. There is data to support the safety of MARS and studies that have shown improvement in ammonia levels, bilirubin, and creatinine. Generally, studies are limited by sample size.
It should be noted that studies on ECLS like plasmapheresis and MARS are frequently biased as they are evaluating the sickest patients who are quickly listed for LT. Given that transplant is a realistic and common outcome for a child listed with high priority, the results of these studies therefore do not give us optimal information on whether these approaches are helpful.
Lastly, renal replacement therapy may be indicated in some patients in liver failure who also develop renal failure, hyperammonemia, fluid overload, and hepatorenal syndrome. While there is not an established ammonia threshold to initiate continuous renal replacement therapy (RRT) in children or adults, levels > 200 µmol/L are associated with poor outcomes, and rapidly rising level should thus prompt consideration. Other considerations for RRT include worsening HE, and hyponatremia refractory to medical treatment.
Take Home Points
- Pediatric acute liver failure is a rapidly evolving disease state that requires prompt recognition and management at a pediatric liver transplant center.
- Management of PALF is largely supportive, but targeted therapies are indicated for specific etiologies; thus, a broad workup is essential and use of extracorporeal liver support systems to help bridge the patient to recovery or liver transplantation should be considered.
- The cause of PALF is never found in 30-50% of cases.
