Inborn Errors of Metabolism- the Great Hepatic Masquerader
What is Gaucher Disease?
Gaucher Disease is a lysosomal storage disease caused by glucocerebrosidase enzyme deficiency resulting in accumulation of glucocerebroside in lysosomes of macrophages in various tissues. Gaucher Disease is a multisystemic disorder, which may manifest at any age from infancy to adulthood. There are 3 types of Gaucher Disease. Types 2 and 3 have primary neurologic disease and commonly manifest in childhood. Type 1 has no primary central nervous system manifestations and may mimic primary liver disease with patients having elevated liver enzymes, hepatosplenomegaly, thrombocytopenia, bleeding diathesis and pathologic fractures. Gaucher Disease type 1 is an example of how patients with metabolic disorders may seek care initially with a hepatologist.
When should I suspect a metabolic disorder mimicking liver disease?
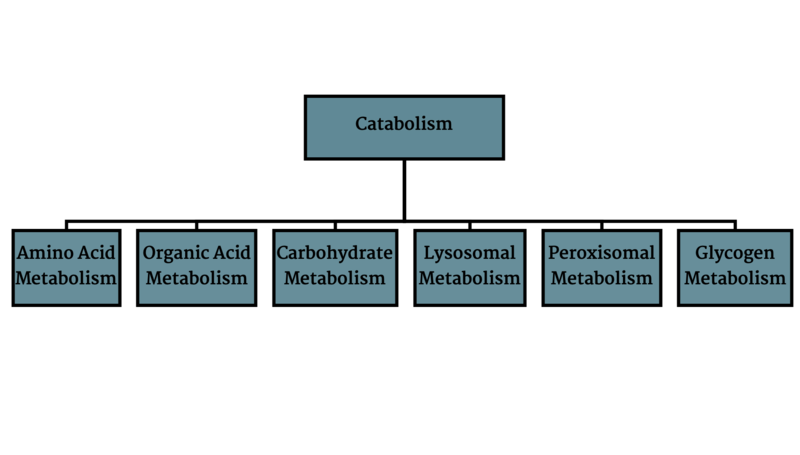
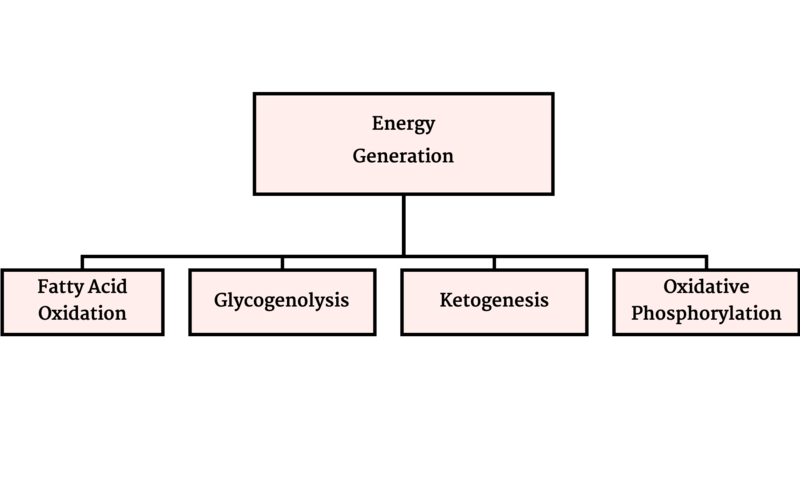
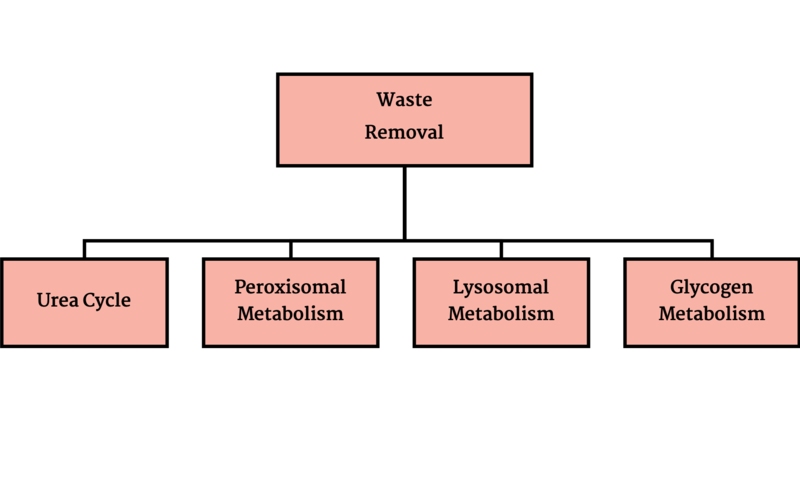
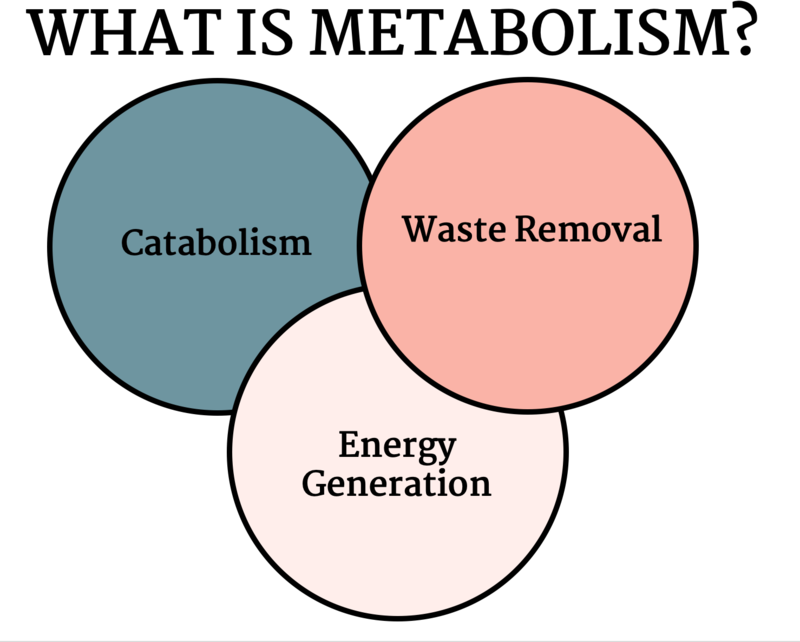
Metabolic disorders result from disruption of catabolism (Figure 1), energy generation (Figure 2) or waste removal (Figure 3) that are all intertwined (Figure 4).
Figure 1: Catabolism
Figure 2- Energy Generation
Figure 3- Waste Removal
Figure 4 -
Many of these disorders have hepatic involvement that may manifest from infancy to early adulthood. This presents a difficulty in defining these disorders as many pediatric cholestatic diseases can be considered metabolic disorders due to inability to synthesize or catabolize cellular products. For instance, Alpha 1 Antitrypsin deficiency (A1AT) & disorders of bile acid synthesis are defined as cholestatic liver disorders but result from accumulation of toxic byproducts. Clinicians should have a high index of suspicion for a metabolic disorder if there are extra hepatic manifestations, for example:
- Aversion to certain foods- females with Ornithine Transcarbamylase deficiency (x-linked inheritance) may have aversion to high protein foods; alcohol may precipitate acute intermittent porphyria crisis.
- Hypoglycemia without other indication of liver synthetic dysfunction- can be seen in glycogen storage diseases or mitochondrial hepatopathies.
- Intellectual disability- many patients with metabolic disorders have intellectual disability and neurological manifestations.
- Chronic lactic acidosis
- Multiple organ involvement
See this review to learn more on how to develop a differential diagnosis for metabolic liver diseases.
Evaluating a patient with suspected metabolic liver disorder?
There is an arsenal of tests to assess biochemical pathways of the liver. After taking a thorough history and careful physical exam the clinician should determine what test will have the highest diagnostic yield and best targeted molecular testing.
- Plasma amino acids- different disorders will have certain patterns of elevated amino acids, for example- urea cycle disorders will have elevated glutamine and mitochondrial disorders will have elevated alanine. This test is not sensitive. If there is a high index of suspicion, further molecular testing should be pursued.
- Acylcarnitine profile- these are carbon chains conjugated to carnitine to facilitate entry to mitochondria. Measuring serum concentrations of these are helpful in determining different etiologies associated with energy metabolism such as fatty acid oxidation defects or organic acidemias.
- Urine Organic Acid profile- elevated succinylacetone will be indicative of tyrosinemia type I. This test is useful for diagnosis of fatty acid oxidation defects, organic acidemias, mitochondrial hepatopathies and aminoacidopathies.
- Lactate to pyruvate ratio- helpful in diagnosis of mitochondrial hepatopathies or disorders of pyruvate metabolism. However, Feldman et al. found that in patients with pediatric acute liver failure it is non-specific in distinguishing cases of mitochondrial hepatopathies from other etiologies.
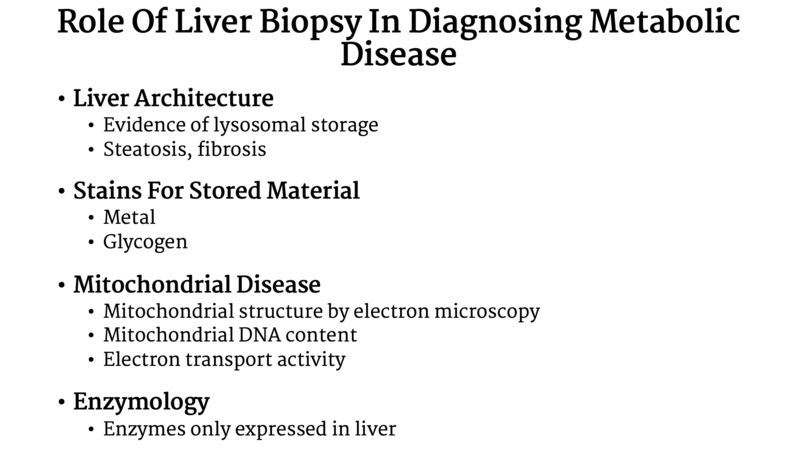
Ferreira et al. provided an extensive list of inborn errors of metabolism with associated liver involvement and suggested testing, which can be found here. An important rule is to never hesitate to reach out to your local friendly genetic/metabolic specialist to help with interpreting abnormal results. They can help determine what is the best next step for evaluating etiology. Always discuss with a metabolism colleague if tissue sample is needed for testing (Table 1) prior to preforming a liver biopsy.
Table 1: Liver biopsy in Metabolic Disease
Treatment of hepatic metabolic disorders
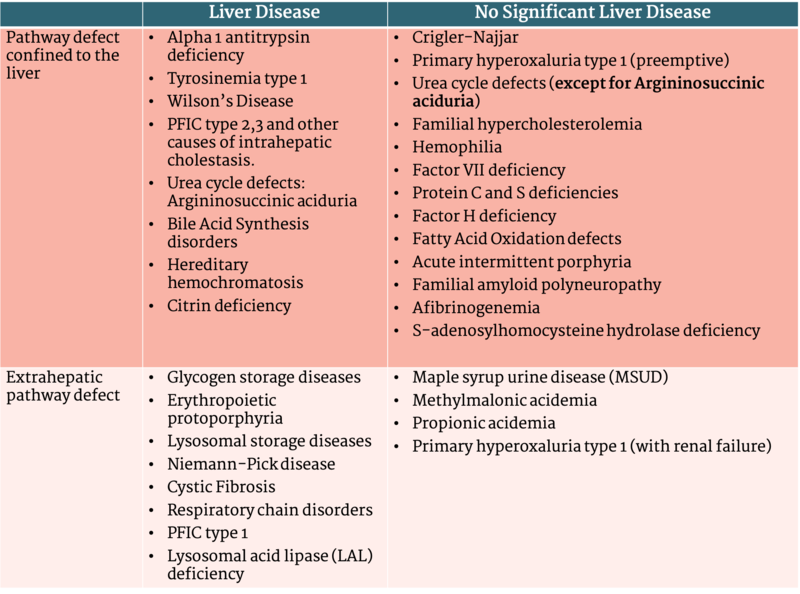
The mainstay of therapy in a patient with inborn errors of metabolism is reducing ingestion and generation of the toxic products that cause disease. This can often be achieved by dietary restriction, medications that decrease the production of toxic metabolic byproducts, or, in rare cases, enzyme replacement therapy. For certain disorders liver transplantation (LT) can provide definitive treatment, and for others it can significantly decrease the chance for metabolic crisis and improve the quality of life for patients. Disorders of inborn errors of metabolism (IEM) are a common indication for LT (17% of pediatric LT in the USA). In these patients, hepatic function is normal aside from the pathway involved in the disease. Liver transplantation is curative of the disorder in many cases by replacing the enzymatic or transporter defect. Indications for liver transplantation can be categorized by metabolic pathways confined to the liver or extrahepatic defects. United Network of Organ Sharing (UNOS) distinguishes between IEM and other metabolic disorders resulting in cholestasis such as those of bile acid metabolism or transport (for example Progressive Familial Intrahepatic Cholestasis or Alpha 1 Antitrypsin Deficiency). This table adapted from McKiernan et al. (Table 2) includes a broad definition of metabolic disorders (including cholestatic genetic disorders) and is useful in categorizing indication for liver transplantation.
Table 2 - Categories of Metabolic Disease When Considering Liver Transplantation
Table adapted from McKiernan et al. Liver Transplantation 2019
A challenging group of disorders are mitochondrial hepatopathies, in which liver transplantation may not treat ongoing extrahepatic manifestations of the disease. Decision to pursue transplantation should involve all interdisciplinary stakeholders. When there is extrahepatic involvement of the enzymatic defect, the transplant team and family need to weigh the risk of transplantation with the possibility of only partial amelioration of disease while using an organ from a limited pool of deceased donors in the US. The option of living related-donor transplantation provides a therapeutic option that has no effect on deceased organ allocation. Interestingly, outcomes for most metabolic disorders are comparable between deceased donor and living donor transplantation, despite relatives being carriers of disease causing mutations for these disorders.