Back to Basics: The Conundrum of Coagulopathy in Cirrhosis
Learning Points
- Review the basics of hemostasis
- Discuss coagulopathy that occurs in cirrhosis
- Highlight considerations and recommendations for management of periprocedural bleeding risk in patients with cirrhosis
Introduction:
In cirrhosis, impaired hemostasis is mediated by coagulation cascade disturbances, thrombocytopenia, platelet and endothelial dysfunction, and altered fibrinolysis. This results in a “rebalanced state” in which the tendency for bleeding or thrombosis can occur simultaneously. As cirrhosis is a spectrum of disease states ranging from compensated to decompensated disease, the alterations in hemostasis resulting in the rebalanced state can differ. Understanding the stages of hemostasis is important to appreciate how coagulopathy in cirrhosis occurs.
Hemostasis: what is it and who are the players?
Hemostasis is the process through which clot formation results in the cessation of bleeding. It occurs in four stages: i) vasoconstriction, ii) platelet plug formation, iii) coagulation cascade, and iv) fibrinolysis (Figure 1).
i) Vasoconstriction: Blood vessels spasm or vasoconstrict in response to endothelial injury to slow the flow of blood. Endothelial injury could occur in setting of stress (sepsis, direct shear stress, or chronic inflammatory states like cirrhosis). This process is mediated by endothelin release (secreted by endothelial cells) and nerve reflexes which causes smooth muscle cells to contract.

BioRender.com
ii) Platelet plug formation: Following endothelium disruption in injury, platelet activation, adhesion, and aggregation occur. Von Willebrand Factor (vWF) is secreted by endothelial cells at the site of injury and binds subendothelial collagen and platelets (glycoprotein Ib receptor), activating platelets. Once platelets are activated, changes in glycoprotein IIb/IIIa platelet receptor occurs allowing for fibrinogen to bind. Fibrinogen binding to this receptor promotes platelet aggregation. Ultimately, these steps result in temporary platelet plug formation.
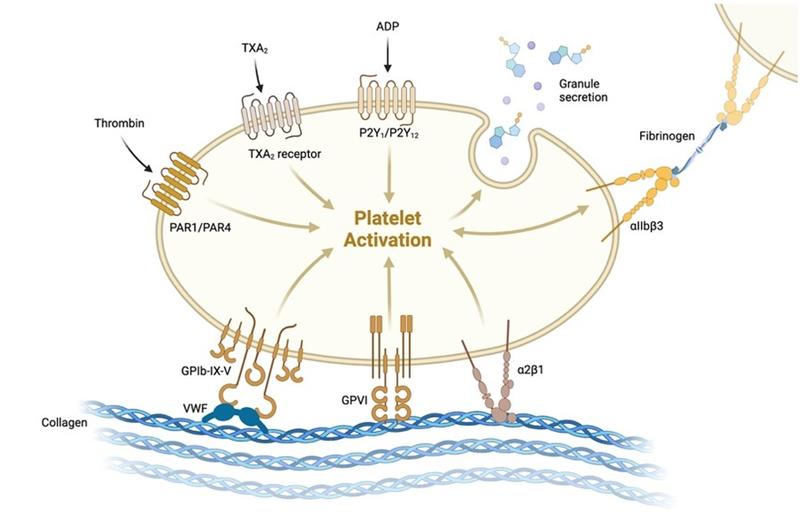
BioRender.com
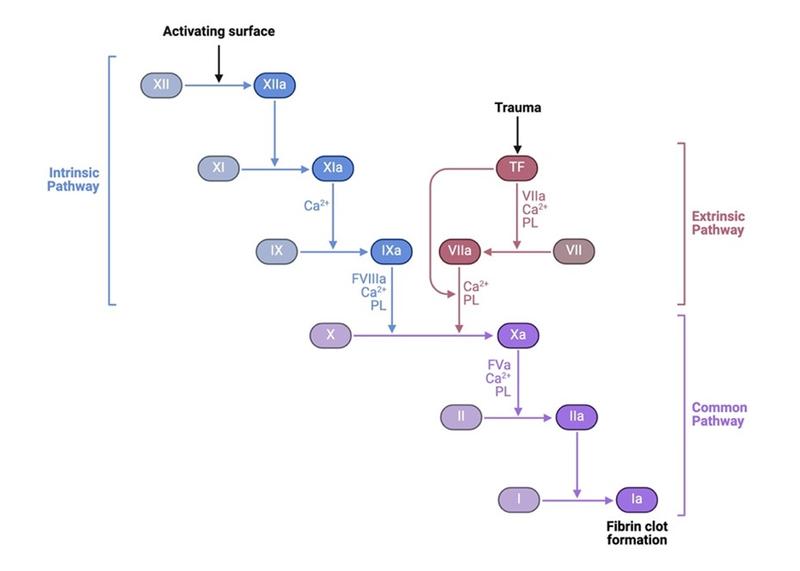
iii) Coagulation cascade: Consists of two pathways, the extrinsic and intrinsic pathways. The extrinsic pathway is activated during the initial vessel injury when tissue factor binds factor VII which binds factor X and leads to activation of thrombin. Thrombin ultimately converts fibrinogen to fibrin which solidifies the platelet plug. More details on the coagulation cascade pathways can be reviewed in this LFN post.
BioRender.com
iv) Fibrinolysis: The process of fibrin breakdown, destabilizing the clot and restoring normal blood flow. Thrombin formation stimulates the release of tissue plasminogen activator (tPA) from endothelial cells. This enzyme catalyzes the transformation of plasminogen to plasmin, which breaks apart fibrin strands.
BioRender.com
** Both the coagulation cascade and fibrinolysis have negative feedback mechanisms which result in appropriate clot formation during the time of injury and restoration of normal blood flow following tissue regeneration.
What happens with hemostasis in cirrhosis?
In cirrhosis, a combination of pro-bleeding and pro-thrombotic changes impact each stage of hemostasis and contribute to a “rebalanced state”. The physiology of these changes occurring at each stage in hemostasis can be reviewed in this LFN post.
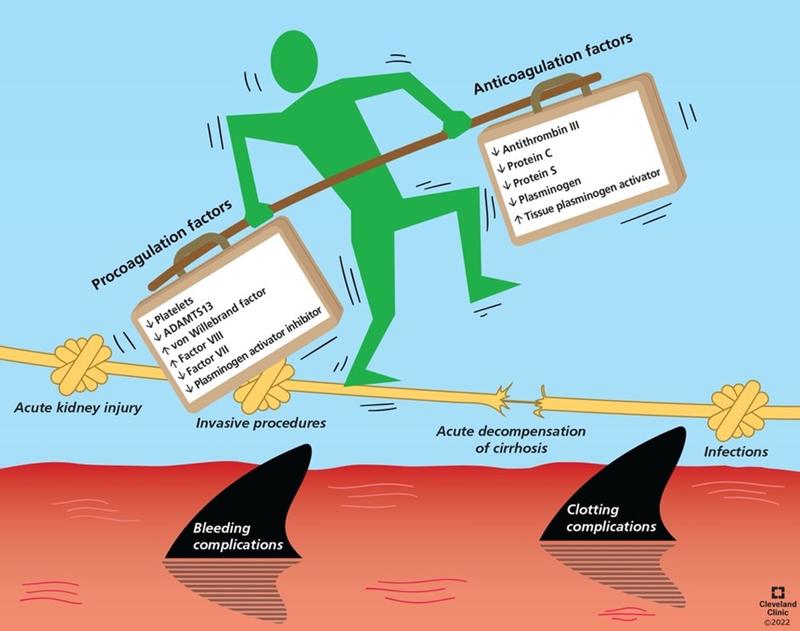
Figure 1. In cirrhosis, splenomegaly and synthetic liver dysfunction result in thrombocytopenia and coagulation factor deficiencies respectively which favor bleeding. Decreased antifibrinolytic proteins in cirrhosis also favors bleeding. Endothelial dysfunction results in elevated vWF and factor VIII and decreased plasminogen, increasing coagulability. This process is dynamic and balance can be tipped by acute kidney injury, acute decompensation of cirrhosis, or stress (invasive procedures, infections). Source: Singh et al., Cleveland Clinic Journal of Medicine.
What are viscoelastic assays and how are they used in cirrhosis?
Coagulation parameters including INR and platelet counts, can be abnormal in patients with cirrhosis, yet it is well known that they don’t accurately reflect bleeding risk. Viscoelastic assays, thromboelastography (TEG) and rotational thromboelastography (ROTEM) have been used to stratify risk of bleeding in surgery and critical care literature for more than 30 years and reduce the use of prophylactic transfusions. Since each individual patient with cirrhosis will have their own unique abnormalities within the various pathways that cause coagulopathy, these tests are thought to help identify more physiologically-accurate abnormalities in real-time to guide management. While these assays may have some utility in the setting of acute bleeding, they have not been clinically validated in patients with cirrhosis.
Periprocedural Considerations in the Management of Coagulopathy
While homeostasis is “rebalanced” in cirrhosis, the risk of bleeding in the setting of portal hypertension as well as the risk of portal or splenic vein thrombosis are common. Thus, it can be difficult to assess the benefit or risk of transfusion, particularly in the periprocedural setting.
The AASLD, AGA, and EASL societies have contributed guidelines providing expert recommendations for the management of bleeding risk and thrombosis with coagulopathy in cirrhosis. The recommendations provide evidence supporting clinical decision-making practices for assessing bleeding risk and transfusion of blood products before procedures.
Before a procedure, the following factors that modulate bleeding risk should be considered:
- What is the bleeding risk of the procedure? (see the 2020 AASLD practice guidelines with bleeding risk stratification for common procedures).
- Where is the procedure being performed, outpatient or inpatient? For outpatient paracenteses (diagnostic and therapeutic), checking coagulation studies is not routinely recommended.
- Does the patient have compensated or decompensated cirrhosis? Acute on chronic liver failure (ACLF)? Patients with decompensated cirrhosis and ACLF have an increased risk of bleeding.
- Are there other systemic factors that could increase bleeding risk (i.e. acute kidney injury or chronic kidney disease and infection)?
- Are antiplatelet/anticoagulant medications being used concomitantly?
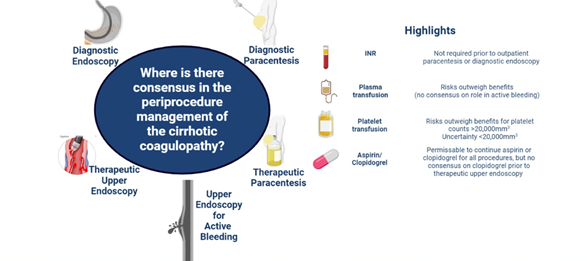
Figure 2. Consensus from a multi-society panel of experts that summarize practice recommendations for periprocedural management of coagulopathy in cirrhosis in the setting of endoscopy and paracenteses. Source: Tapper et al., Hepatology.
Take-home points:
- In cirrhosis, the pathophysiology of hemostasis is altered such that there is a balance of factors promoting both bleeding and coagulation.
- Coagulopathy in patients with cirrhosis is complicated with a variety of pathways that can increase risk of bleeding and increase risk of clotting. Therefore, thrombocytopenia and elevated INR alone do not accurately reflect bleeding risk in patients with cirrhosis.
- Viscoelastic assays can be used to reduce prophylactic transfusions and provide in vivo insight into coagulopathy favoring bleeding or hypercoagulability; however, they have not been clinically validated for use in procedure-related bleeding risk assessment.
- Periprocedural assessment should consider modulators that increase the risk of bleeding including AKI/CKD, infection/sepsis, and ACLF.